Above, aromatic compounds were defined as compounds that resemble benzene. But what specific properties of benzene must a compound have in order for it to be classified as aromatic? In addition to compounds containing benzene rings, there are many other substances that are called aromatics, although some of them bear little resemblance to benzene in appearance.
What properties are characteristic of all aromatic compounds?
From an empirical point of view, aromatic compounds are compounds whose molecular formula corresponds to a high degree of unsaturation and which, nevertheless, do not undergo the addition reactions usually characteristic of unsaturated compounds. Instead of addition reactions, these aromatic compounds often undergo electrophilic substitution reactions, like benzene. Along with inertness in addition reactions, the unusual stability of these compounds also manifests itself - low values of the heats of hydrogenation and combustion. Aromatic compounds have a cyclic structure - usually containing five-, six-, or seven-membered rings - and when studied by physical methods their molecules are found to be flat (or almost flat). The protons in these compounds have approximately the same chemical shift values in the NMR spectra (Section 13.18) as the protons in benzene and its derivatives.
From a theoretical point of view, for a compound to be aromatic, its molecule must contain a cyclic system of delocalized electrons above and below the plane of the molecule; Moreover, the cloud of -electrons must contain -electrons. In other words, for the degree of stability that is characteristic of aromatic compounds, delocalization alone is not enough. The molecule must contain a strictly defined number of electrons - 2, or 6, or 10, etc. This requirement, known as the Hückel rule or rule (named after Erich Hückel, Institute for Theoretical Physics, Stuttgart), is based on quantum mechanics and is associated with the requirements for filling the various orbitals that form the -cloud. The validity of Hückel's rule is well confirmed by facts.
Let's look at some data confirming Hückel's rule. Benzene has six -electrons, an aromatic sextet; the number six is the Hückel number for In addition to benzene and similar substances (naphthalene, anthracene, phenanthrene, Chapter 35), we will also meet a large number heterocyclic compounds that have distinct aromatic properties; as will be shown, to similar
Aromatic heterodicles include those compounds in which an aromatic sextet can occur.
As other examples, consider the following six compounds, each of which has only one resonance structure:

Each molecule is a hybrid of five or six equivalent structures containing a charge or unpaired electron on each of the carbon atoms. However, of these six compounds, only two have unusually high stability: the cyclopentadienyl anion and the cycloheptatrienyl cation (tropylium ion).
Cyclopentadiene is an unusually strong acid for a hydrocarbon, indicating increased stability of the anion formed when a hydrogen ion is abstracted. (Cyclopentadiene is a much stronger acid than cycloheptatriene, although the latter's anion is stabilized by the resonance of seven structures.) Dicyclopentadienyliron (ferrocene) is a stable molecule having a sandwich structure in which the iron atom is sandwiched between two five-membered rings. All carbon–carbon bonds are 1.4 A long. Ferrocene rings undergo typical aromatic substitution reactions - sulfonation and the Friedel-Crafts reaction.

Among cycloheptatrienyl derivatives, it is the cation that has unusual properties. Tropylium bromide melts, is highly soluble in water, but is insoluble in non-polar solvents and precipitates instantly upon action. This behavior is unusual for organic bromide and suggests that even in the solid state we are dealing with an ionic compound in which the cation is actually a stable carbonium ion.
Let's consider electronic configuration cyclopentadienyl anion (Fig. 10.5). Each carbon atom is trigonally hybridized and bonded by -bonds to two other carbon atoms and one hydrogen atom. The ring of the cyclopentadienyl anion is a regular pentagon, the angles of which are ; some instability resulting from imperfect overlap (angular stress) is more than compensated for by the resulting delocalization. Four carbon atoms have one electron in each -orbital, the fifth carbon atom (the one that has lost a proton, but in reality, of course, indistinguishable from the others) has a pair of electrons.

Rice. 10.5. Cyclopentadienyl anion. a - two electrons in the a-orbital of one of the carbon atoms, one electron in the -orbitals of each of the other carbon atoms; b - overlap of -orbitals with the formation of -bonds; c - clouds above or below the plane of the ring; there are only six -electrons, i.e., an aromatic sextet.
The overlap of -orbitals leads to the formation of -clouds containing a total of six electrons, i.e., an aromatic sextet.
The configuration of the tropylium ion is depicted in a similar way. It is a regular heptagon (angles equal to 128.5° (2.242 rad)]. Six carbon atoms each have one -electron, and the seventh has only an empty -orbital. The result is an aromatic sextet.
The considered ions are most conveniently represented as follows:

The most common systems are those with a Hückel number of six, which is understandable. In order for the atoms of an aromatic ring to have -orbitals, they must be in -hybridization and, therefore, ideally the bond angles should be equal in order for the overlap of the -orbitals to be possible, leading to the formation of an -cloud, the aromatic compound must be planar or almost planar . The number of trigonally hybridized atoms that can form a planar ring without too much angular stress is five, six, or seven, and only a planar ring allows enough overlap to form an -bond. The Hückel number of six corresponds to the number of -electrons that can be provided in common system the specified number of ring atoms. (It is no coincidence that benzene, an example of an aromatic compound, is also an ideal structure: it contains six atoms capable of donating six electrons and forming a hexagon, the angles of which are exactly trigonal.)
Let us now consider what evidence is there that other Hückel numbers are also “magic numbers”? In these cases it cannot be expected that the aromatic character will necessarily manifest itself in extreme stability of the compounds comparable to that of benzene and related compounds. Rings with this number of carbon atoms will be too small or too large to accommodate trigonally hybridized carbon atoms well. Therefore, any stabilization due to aromaticity can be largely compensated for by angular stress or. low degree of overlap of -orbitals or both factors.
Stability should be considered only in a comparative sense, as was done above for cyclopentadienyl and cycloheptatrienyl derivatives, and evidence of aromaticity should be seen in the fact that one or another molecule is more stable than related ones. The rule was fully confirmed experimentally. The current research objective is to clarify a more complex question, namely: at what maximum unfavorable combination of angular stress and electrostatic repulsions due to the presence of multiple charges can aromaticity still be observed?
(see scan)
Aromatic hydrocarbons- compounds of carbon and hydrogen, the molecule of which contains a benzene ring. The most important representatives of aromatic hydrocarbons are benzene and its homologues - products of the replacement of one or more hydrogen atoms in a benzene molecule with hydrocarbon residues.

The structure of the benzene molecule
The first aromatic compound, benzene, was discovered in 1825 by M. Faraday. Its molecular formula was established - C6H6. If we compare its composition with the composition of a saturated hydrocarbon containing the same number of carbon atoms - hexane (C 6 H 14), then we can see that benzene contains eight less hydrogen atoms. As is known, the appearance of multiple bonds and cycles leads to a decrease in the number of hydrogen atoms in a hydrocarbon molecule. In 1865, F. Kekule proposed its structural formula as cyclohexanthriene-1,3,5.

Thus, a molecule corresponding to the Kekulé formula contains double bonds, therefore, benzene must be unsaturated, i.e., easily undergo addition reactions: hydrogenation, bromination, hydration, etc.

However, data from numerous experiments have shown that benzene undergoes addition reactions only under harsh conditions(at high temperatures and lighting), resistant to oxidation. The most characteristic reactions for it are substitution reactions Therefore, benzene is closer in character to saturated hydrocarbons.
In an attempt to explain these discrepancies, many scientists have proposed various options benzene structure. The structure of the benzene molecule was finally confirmed by the reaction of its formation from acetylene. In reality, the carbon-carbon bonds in benzene are equivalent, and their properties are not similar to those of either single or double bonds.
Currently, benzene is denoted either by the Kekule formula or by a hexagon in which a circle is depicted.

So what is special about the structure of benzene?
Based on research data and calculations, it was concluded that all six carbon atoms are in a state of sp 2 hybridization and lie in the same plane. The unhybridized p-orbitals of the carbon atoms that make up the double bonds (Kekule formula) are perpendicular to the plane of the ring and parallel to each other.
They overlap each other, forming a single π-system. Thus, the system of alternating double bonds depicted in Kekulé’s formula is a cyclic system of conjugated, overlapping π bonds. This system consists of two toroidal (donut-like) regions of electron density lying on either side of the benzene ring. Thus, it is more logical to depict benzene as a regular hexagon with a circle in the center (π-system) than as cyclohexanthriene-1,3,5.
The American scientist L. Pauling proposed to represent benzene in the form of two boundary structures that differ in the distribution of electron density and constantly transform into each other:

Bond length measurements confirm this assumption. It was found that all C-C bonds in benzene have the same length (0.139 nm). They are slightly shorter than single ones C-C connections(0.154 nm) and longer than double ones (0.132 nm).
There are also compounds whose molecules contain several cyclic structures, for example:

Isomerism and nomenclature of aromatic hydrocarbons
For benzene homologues isomerism of the position of several substituents is characteristic. The simplest homolog of benzene is toluene(methylbenzene) - has no such isomers; the following homologue is presented as four isomers:

The basis of the name of an aromatic hydrocarbon with small substituents is the word benzene. The atoms in the aromatic ring are numbered, starting from senior deputy to junior:

If the substituents are the same, then numbering is carried out along the shortest path: for example, substance:

called 1,3-dimethylbenzene, not 1,5-dimethylbenzene.
According to the old nomenclature, positions 2 and 6 are called orthopositions, 4 - para-positions, 3 and 5 - meta-positions.


Physical properties of aromatic hydrocarbons
Benzene and its simplest homologues under normal conditions - very toxic liquids with a characteristic unpleasant odor. They dissolve poorly in water, but well in organic solvents.
Chemical properties of aromatic hydrocarbons

Substitution reactions. Aromatic hydrocarbons undergo substitution reactions.
1. Bromination. When reacting with bromine in the presence of a catalyst, iron (III) bromide, one of the hydrogen atoms in the benzene ring can be replaced by a bromine atom:

2. Nitration of benzene and its homologues. When an aromatic hydrocarbon reacts with nitric acid in the presence of sulfuric acid (a mixture of sulfuric and nitric acids called a nitrating mixture), the hydrogen atom is replaced by a nitro group - NO 2:

By reducing nitrobenzene we obtain aniline- a substance that is used to obtain aniline dyes:

This reaction is named after the Russian chemist Zinin.
Addition reactions. Aromatic compounds can also undergo addition reactions to the benzene ring. In this case, cyclohexane and its derivatives are formed.
1. Hydrogenation. Catalytic hydrogenation of benzene occurs at a higher temperature than the hydrogenation of alkenes:

2. Chlorination. The reaction occurs when illuminated with ultraviolet light and is free radical:

Chemical properties of aromatic hydrocarbons - summary



Benzene homologues
The composition of their molecules corresponds to the formula CnH2n-6. The closest homologues of benzene are:
All benzene homologues following toluene have isomers. Isomerism can be associated both with the number and structure of the substituent (1, 2), and with the position of the substituent in the benzene ring (2, 3, 4). Connections general formula C 8 H 10 :

According to the old nomenclature used to indicate the relative location of two identical or different substituents on the benzene ring, the prefixes are used ortho-(abbreviated o-) - substituents are located on neighboring carbon atoms, meta-(m-) - through one carbon atom and pair-(n-) - substituents opposite each other.
The first members of the homologous series of benzene are liquids with a specific odor. They are lighter than water. They are good solvents. Benzene homologues undergo substitution reactions:
bromination:

nitration:

Toluene is oxidized by permanganate when heated:

Reference material for taking the test:
Mendeleev table
Solubility table
ARENES
Aromatic hydrocarbons (arenes) – cyclic hydrocarbons, united by the concept of aromaticity, which determines general signs in structure and chemical properties.
Classification
Based on the number of benzene rings in the molecule, arenes are divided into on the:
mononuclear
multi-core

Nomenclature and isomerism
The structural ancestor of benzene series hydrocarbons is benzene C 6 H 6 from which the systematic names of homologues are derived.
For monocyclic compounds, the following non-systematic (trivial) names are retained:

The position of the substituents is indicated in the smallest numbers (the direction of numbering does not matter),
|
|
|
and for di-substituted compounds you can use the notation ortho, meta, pair.
|
|
|
|
If there are three substituents in the ring, they should receive the lowest numbers, i.e. the row “1,2,4” has an advantage over “1,3,4”.


1,2-dimethyl-4-ethylbenzene (correct name) 3,4-dimethyl-1-ethylbenzene (incorrect name)
The isomerism of monosubstituted arenes is due to the structure of the carbon skeleton of the substituent; in di- and polysubstituted benzene homologues, additional isomerism is added, caused by the different arrangement of substituents in the nucleus.
Isomerism of aromatic hydrocarbons with the composition C 9 H 12:
|
|
|
|
|
Physical properties
The boiling and melting points of arenes are higher than those of alkanes, alkenes, alkynes, they are slightly polar, insoluble in water and highly soluble in non-polar organic solvents. Arenas are liquids or solids that have specific odors. Benzenes and many condensed arenes are toxic, some of them exhibit carcinogenic properties. Intermediate products of the oxidation of condensed arenes in the body are epoxides, which either themselves directly cause cancer or are precursors of carcinogens.
Getting arenas
Many aromatic hydrocarbons have important practical significance and are produced on a large industrial scale. A number of industrial methods are based on the processing of coal and oil.
Oil consists mainly of aliphatic and alicyclic hydrocarbons; to convert aliphatic or acyclic hydrocarbons into aromatic ones, methods for aromatizing oil have been developed, the chemical basis of which was developed by N.D. Zelinsky, B.A. Kazansky.
1. Cyclization and dehydrogenation:
2. Hydrodesmethylation:

3. Benzene homologues are prepared by alkylation or acylation followed by reduction of the carbonyl group.
a) Friedel-Crafts alkylation:

b) Friedel-Crafts acylation:

4. Preparation of biphenyl by the Wurtz-Fitting reaction:
5. Preparation of diphenylmethane by the Friedel-Crafts reaction:
Structure and Chemical properties.
Aromaticity criteria:
Based on theoretical calculations and experimental studies of cyclic conjugated systems, it was found that a compound is aromatic if it has:
- Flat cyclic σ-skeleton;
- Conjugate closed π -electronic system, covering all atoms of the cycle and containing 4n + 2, where n = 0, 1, 2, 3, etc. This formulation is known as Hückel's rule. Aromaticity criteria allow one to distinguish conjugated aromatic systems from all others. Benzene contains a sextet of π electrons and follows Hückel's rule at n = 1.
What does aromaticity give:
Despite the high degree of unsaturation, aromatic compounds are resistant to oxidizing agents and temperature, and they are more prone to undergo substitution reactions rather than addition reactions. These compounds have increased thermodynamic stability, which is ensured by the high conjugation energy of the aromatic ring system (150 kJ/mol); therefore, arenes preferably enter into substitution reactions, as a result of which they retain aromaticity.
Mechanism of electrophilic substitution reactions in the aromatic ring:
The electron density of the π-conjugated system of the benzene ring is a convenient target for attack by electrophilic reagents.
Typically, electrophilic reagents are generated during a reaction using catalysts and appropriate conditions.
E – Y → E δ + – Y δ - → E + + Y -
Formation of a π-complex. The initial attack by the electrophile of the π-electron cloud of the ring leads to coordination of the reagent with the π-system and the formation of a donor-acceptor type complex called π-complex. The aroma system is not disrupted:

Formation of the σ-complex. The limiting stage, in which the electrophile forms a covalent bond with a carbon atom due to two electrons of the π-system of the ring, which is accompanied by the transition of this carbon atom from sp 2 - V sp 3 - hybrid state and aromatic disruption, the molecule turns into a carbocation.

Stabilization of the σ-complex. It is carried out by abstraction of a proton from the σ-complex using a base. In this case, due to the two electrons of the breaking covalent bond C–H, the closed π-system of the ring is restored, i.e. the molecule returns to the aromatic state:

Effect of substituents on reactivity and orientation of electrophilic substitution
Substituents on the benzene ring disrupt the distribution uniformity π- electron cloud of the ring and thereby influence the reactivity of the ring.
- Electron-donating substituents (D) increase the electron density of the ring and increase the rate of electrophilic substitution; such substituents are called activating.
- Electron-withdrawing substituents (A) lower the electron density of the ring and reduce speed reaction, are called decontaminating.
Cyclic conjugated systems are of great interest as a group of compounds with increased thermodynamic stability compared to conjugated open systems. These compounds also have other special properties, the totality of which is combined general concept aromaticity. These include the ability of such formally unsaturated compounds to undergo substitution rather than addition reactions, resistance to oxidizing agents and temperature.
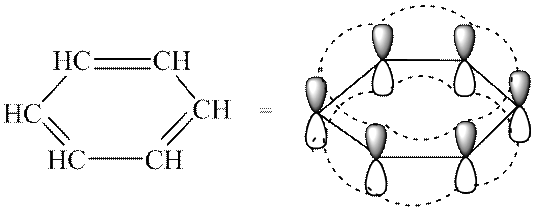
Typical representatives of aromatic systems are arenes and their derivatives. The features of the electronic structure of aromatic hydrocarbons are clearly manifested in the atomic orbital model of the benzene molecule. The benzene framework is formed by six sp 2 -hybridized carbon atoms. All σ bonds (C-C and C-H) lie in the same plane. Six unhybridized p-AOs are located perpendicular to the plane of the molecule and parallel to each other (Fig. 3a). Each R-AO can equally overlap with two neighboring ones R-AO. As a result of such overlap, a single delocalized π-system arises, the highest electron density in which is located above and below the plane of the σ-skeleton and covers all carbon atoms of the cycle (see Fig. 3, b). The π-Electron density is evenly distributed throughout the cyclic system, which is indicated by a circle or dotted line inside the cycle (see Fig. 3, c). All bonds between carbon atoms in the benzene ring have the same length (0.139 nm), intermediate between the lengths of single and double bonds.
Based on quantum mechanical calculations, it was established that for the formation of such stable molecules, a flat cyclic system must contain (4n + 2) π electrons, where n= 1, 2, 3, etc. (Hückel's rule, 1931). Taking these data into account, the concept of “aromaticity” can be specified.
Aroma systems (molecules)– systems that meet aromaticity criteria :
1) the presence of a flat σ-skeleton consisting of sp 2 -hybridized atoms;
2) delocalization of electrons, leading to the formation of a single π-electron cloud covering all atoms of the cycle (cycles);
3) compliance with E. Hückel’s rule, i.e. the electron cloud should contain 4n+2 π-electrons, where n=1,2,3,4... (usually the number indicates the number of cycles in the molecule);
4) high degree thermodynamic stability (high conjugation energy).
Rice. 3. Atomic orbital model of the benzene molecule (hydrogen atoms omitted; explanation in text)
Stability of coupled systems. The formation of a conjugated and especially aromatic system is an energetically favorable process, since this increases the degree of overlap of orbitals and delocalization (dispersal) occurs. R-electrons. In this regard, conjugated and aromatic systems have increased thermodynamic stability. They contain a smaller supply of internal energy and in the ground state occupy a lower energy level compared to non-coupled systems. From the difference between these levels, one can quantify the thermodynamic stability of the conjugated compound, i.e., its conjugation energy (delocalization energy). For butadiene-1,3 it is small and amounts to about 15 kJ/mol. As the length of the conjugated chain increases, the conjugation energy and, accordingly, the thermodynamic stability of the compounds increase. The conjugation energy for benzene is much higher and amounts to 150 kJ/mol.
Examples of non-benzenoid aromatic compounds:
Pyridine By electronic structure resembles benzene. All carbon atoms and the nitrogen atom are in a state of sp 2 hybridization, and all σ bonds (C-C, C-N and C-H) lie in the same plane (Fig. 4, a). Of the three hybrid orbitals of the nitrogen atom, two are involved in the formation
Rice. 4. Pyridine nitrogen atom (A), (b) and conjugated system in the pyridine molecule (c) ( S-N connections omitted to simplify the figure)
σ bonds with carbon atoms (only the axes of these orbitals are shown), and the third orbital contains a lone pair of electrons and is not involved in the formation of the bond. A nitrogen atom with this electron configuration is called pyridine.
Due to the electron located in the unhybridized p-orbital (see Fig. 4, b), the nitrogen atom participates in the formation of a single electron cloud with R-electrons of five carbon atoms (see Fig. 4, c). Thus, pyridine is a π,π-conjugated system and satisfies the criteria for aromaticity.
As a result of greater electronegativity compared to the carbon atom, the pyridine nitrogen atom lowers the electron density on the carbon atoms of the aromatic ring, therefore systems with a pyridine nitrogen atom are called π-insufficient. In addition to pyridine, an example of such systems is pyrimidine, containing two pyridine nitrogen atoms.
Pyrrole also refers to aromatic compounds. The carbon and nitrogen atoms in it, as in pyridine, are in a state of sp2 hybridization. However, unlike pyridine, the nitrogen atom in pyrrole has a different electronic configuration (Fig. 5, a, b).
Rice. 5. Pyrrole nitrogen atom (A), distribution of electrons among orbitals (b) and the conjugated system in the pyrrole molecule (c) (C-H bonds are omitted to simplify the figure)
On unhybridized R The -orbital of the nitrogen atom contains a lone pair of electrons. She is involved in pairing with R-electrons of four carbon atoms to form a single six-electron cloud (see Fig. 5, c). Three sp 2 hybrid orbitals form three σ bonds - two with carbon atoms, one with a hydrogen atom. The nitrogen atom in this electronic state is called pyrrole.
Six-electron cloud in pyrrole thanks to p,p-conjugation is delocalized on five ring atoms, so pyrrole is π-excess system.
IN furane And thiophene the aromatic sextet also includes a lone pair of electrons from the unhybridized p-AO of oxygen or sulfur, respectively. IN imidazole And pyrazole The two nitrogen atoms make different contributions to the formation of a delocalized electron cloud: the pyrrole nitrogen atom supplies a pair of π electrons, and the pyridine nitrogen atom supplies one p electron.
It also has aromatic properties purine, representing a condensed system of two heterocycles - pyrimidine and imidazole.
The delocalized electron cloud in purine includes 8 π double bond electrons and a lone pair of electrons from the N=9 atom. The total number of electrons in conjugation, equal to ten, corresponds to the Hückel formula (4n + 2, where n = 2).
Heterocyclic aromatic compounds have high thermodynamic stability. It is not surprising that they serve as structural units of the most important biopolymers - nucleic acids.
In organic chemistry, such a concept as aromaticity some organic compounds. The term "aromaticity" is associated primarily with benzene, its homologues and numerous derivatives. This term refers exclusively to the structure of the molecules of these substances, their properties, but has nothing to do with their smell. True, the first aromatic compounds probably had a pleasant smell (some natural ethers, fragrant resins, such as incense, etc.).
Aromaticity - a common feature of some cyclic organic compounds that have a set of special properties.
The presence of a single closed system of π-electrons in a molecule - the main sign of aromaticity.
Aromatic compounds obey the rule E. Hückel (1931):
Planar monocyclic compounds having a conjugated system of π-electrons can be aromatic if the number of these electrons is 4n+2 (where n = 0,1,2,3, 4, etc., i.e. the number of π-electrons in a molecule can be 2, 6, 10, 14, 18, etc.).
These features determine all the most important physical and chemical properties of aromatic compounds. For example, they undergo predominantly substitution reactions (mainly electrophilic) rather than addition reactions (despite formal unsaturation). Aromatic compounds are highly resistant, for example, to oxidizing agents. Their molecules have a flat structure. If this requirement is not met, then the parallelism of the axes of the 2p orbitals in the molecule is disrupted, which leads to the elimination of conjugation and, as a consequence, to a violation of the uniformity of the π-electron density in the system.
Nomenclature
The systematic name of all aromatic hydrocarbons is arenas , and benzene - benzene . Benzene homologues are considered as substituted benzenes and numbers indicate the position of the substituents. However, systematic nomenclature allows the name "benzene", and for some homologues of benzene - trivial names: vinylbenzene (I) is called styrene, methylbenzene (II) - toluene, dimethylbenzene (III) - xylene, isopropylbenzene (IV) - cumene, methoxybenzene (V) - anisole etc.:
Aromatic radicals have common name - Arils(Ar). Radical C 6 H 5 - called phenyl(from the old name for benzene - “hair dryer”).
Isomerism.
The general formula of benzene homologues is C n H 2 n -6. All six hydrogen atoms in a benzene molecule are identical, and when one of them is replaced by the same radical, the same compound is formed. Therefore, monosubstituted benzene has no isomers. For example, there is only one methylbenzene:

When two hydrogen atoms are replaced by methyl groups, three isomers are formed - xylenes, which differ from each other in the arrangement of substituents in the ring:



ortho-dimethylbenzene, meta-dimethylbenzene, pair-dimethylbenzene,
or 1,2-dimethylbenzene or 1,3-dimethylbenzene or 1,4-dimethylbenzene
(O-xylene) ( m-xylene) ( P-xylene)
Instead of the letter designation ( ortho-, meta-, para-, or abbreviated: o-, m-, p-) you can use digital: 1,2-, 1,3-, 1,4-. Isomers may differ in the nature of their substituents:


propylbenzene isopropylbenzene