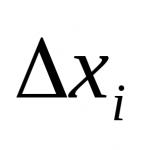Under Sustainability disperse system understand the constancy over time of its state and basic properties: dispersion, uniform distribution of particles in the volume of the medium and the nature of the interaction between particles. The stability of dispersed systems is divided into sedimentation (kinetic), aggregative and phase (condensation).
Sedimentation stability characterizes the ability of a disperse system to maintain a uniform distribution of particles in the volume, i.e. resist the action of gravity and the processes of settling or floating of particles.
Aggregative stability is the ability of a system to resist the process of particle enlargement.
With respect to aggregation, disperse systems are divided into the following.
1. Thermodynamically stable, or lyophilic, which spontaneously disperse and exist without additional stabilization (solutions of colloidal surfactants, solutions of polymers, suspensions - clay, soaps, solutions of hydrocarbons, etc.). When these systems form, the Gibbs free energy decreases: D G<0.
2. Fundamentally thermodynamically unstable, or lyophobic systems. Their instability is caused by an excess of surface energy. They cannot be obtained by spontaneous dispersion (sols, suspensions, emulsions). Energy is always spent on their formation: D G>0.
The process of aggregation of dispersed phase particles as a result of loss of aggregation stability is called coagulation .
Phase (condensation) stability refers to the structure and strength of aggregates formed during coagulation of a dispersed system. Condensation-unstable systems form fragile aggregates or loose sediments, in which the particles lose their mobility, but remain preserved for a long time. This is facilitated interlayers dispersion medium between particles. Units with such structure can again disintegrate into individual particles, i.e. undergo peptization. Condensation-resistant systems are characterized by the formation of aggregates with a strong structure. This is caused by direct phase contact of particles with each other, the process of crystallization, fusion of particles, etc.
The combination of particles can lead to the formation of a continuous structured system with phase stability.
Factors of aggregation sustainability Dispersed systems are divided into thermodynamic and kinetic.
TO thermodynamic factors include the following:
electrostatic- contributes to the creation of electrostatic repulsive forces due to the appearance of a double electric layer (DEL) on the surface of particles;
adsorption-solvation - leads to a decrease in interfacial tension, which prevents rapprochement particles;
entropic - manifests itself in the tendency of particles to be uniformly distributed throughout the volume of the system.
TO kinetic factorssustainability, those that reduce the rate of particle aggregation include the following:
structural-mechanical associated with the formation of protective films on the surface of particles that have elasticity and mechanical strength, resistant to destruction;
hydrodynamic- reduces the speed of particle movement due to changes in the viscosity and density of the dispersion medium.
The theory of stability of hydrophobic colloids was developed by Deryapsh, Landau, and Vervey Overbeck (DLVO theory). Stability of dispersed systems determined by the balance of energy of attraction and repulsion of particles. The energy of attraction is due to intermolecular forces van der Waals and changes back proportional to the square distances between particles. Repulsion energy, by theories DLPO, determined only by the electrostatic component disjoining pressure (repulsion pressure) and decreases With distance according to the exponential law. Depending from the balance of these forces in a thin layer of liquid between approaching particles, either positive disjoining pressure arises, preventing their connection, or negative, leading to thinning of the layer And contact between particles.
The occurrence of disjoining pressure in thin liquid layers is due to the following factors:
1) electrostatic interaction in the layer, caused by the mutual overlap of double electric layers (DEL) - these are repulsive forces with energy U from>0;
2) van der Waals forces of attraction with energy U pr<0;
1) adsorption forces arising when molecular adsorption layers overlap, where an increased concentration creates an osmotic flow towards the film, leading to an increase in the surface energy of the system and, consequently, to repulsion;
2) structural associated with the formation of boundary layers of solvent with a special structure. It is characteristic of lyophilic systems and corresponds to thermodynamic concepts of the adsorption-solvation barrier. The effects are usually positive.
Resultant energy of interparticle interaction U is defined as the sum of two components:
If | U ott | > | U pr |, then repulsive forces predominate, coagulation does not occur, and the sol is aggregatively stable. In the opposite case, the forces of attraction between particles predominate, and coagulation occurs.
Let us consider the quantitative interpretation of these forces.
Electrostatic repulsion between micelles occurs when diffuse layers of counterions overlap. The energy of this interaction:
Where h– distance between particles; is the reciprocal of the thickness of the diffuse layer δ; A– a quantity independent of h and determined by the parameters of the diesel power plant.
The quantities א and A can be calculated based on DES theory.
Calculations show that the repulsion energy decreases:
· at increasing counterion charges And their concentrations;
· at decreasing by absolute valueφ o And z-potential.
From the equation it follows that U ott decreases with increasing distance between particles h according to the exponential law.
Energy of attraction is associated mainly with dispersion interactions between molecules. It can be calculated using the equation
Where A G– Hamaker constant.
From this equation it follows that the energy of attraction changes with increasing distance between particles h inversely proportional to the square of the distance. Thus, attraction decreases relatively slowly with increasing distance. So, with increasing h 100 times the energy of attraction decreases by 10 4 times. At the same time, the repulsion energy decreases by 10 43 times.
The resulting energy of interaction between particles located at a distance h, is determined by the equation:
Dependence of total potential energy interparticle interaction depending on the distance between particles is complex.
General form this dependence U = f(h) presented in Figure 1.
There are three sections on the graph:
1) 0 < h < h 1 . U(h)<0, между частицами преобладают силы притяжения, наблюдается ближний минимум.
U ott → const; U pr → -∞. Coagulation occurs.
2) h 1 <h<h 2 . U(h)>0 – repulsive forces prevail between particles. U ott > | U pr |.
3) h 2 < h < h 3 . U(h)<0 – обнаруживается дальний минимум, однако глубина его невелика.
At h = h 1 , h 2 , h 3 U (h) = 0, i.e., at these distances between particles, the attractive forces are balanced by the repulsive forces.
Thus, if the particles approach at a distance less than h 1, they will inevitably stick together, but for this to happen a potential barrier must be overcome ∆U to. This is possible with sufficient kinetic energy of particles, which on average is close to the product κT.
Let's consider the interaction of two particles. We will consider one particle stationary, and the second one approaching it with an energy equal to κT.
If κT < ∆U etc., the particles will remain at a distance hmin and will be connected to each other through a layer of dispersion medium, i.e. they form a “pair”, but do not directly stick together and do not lose their sedimentation stability. In such cases, the interaction is said to occur at the far minimum.
If ∆ U min < κT << ∆U to, then the particles fly away from each other upon collision. The system is aggregatively stable.
If κT < ∆U to, then slow coagulation occurs.
If κT > ∆U to, then rapid coagulation occurs.
Since the sol is usually considered at a constant temperature, the kinetic energy of the particles remains constant. Therefore, for coagulation to occur, the potential barrier to coagulation must be reduced ∆U to.
Usually, to lower the potential barrier, an electrolyte-coagulant is introduced into the system. The DLFO theory makes it possible to calculate the threshold for rapid coagulation with CB:
Where A, IN– constant quantities that can be calculated;
ε – dielectric constant of the medium;
Z– charge of the coagulant ion;
ē – electron charge.
Lyophobic dispersed systems (sols, emulsions, suspensions) are aggregatively unstable because they have an excess of surface energy. The process of particle enlargement occurs spontaneously, since it leads to a decrease in the specific surface area and a decrease in the surface Gibbs energy.
An increase in particle size can occur due to both coagulation, those. adhesion of particles, so due to isothermal distillation (transfer of matter from small particles to large ones). Coagulation of lyophobic disperse systems can occur under the influence of a number of factors: mechanical influences, light, temperature changes, changes in the concentration of the dispersed phase, and the addition of electrolytes.
There are two types of electrolyte coagulation of colloidal systems: neutralization and concentration.
Neutralizationcoagulation observed at sols with weakly charged particles. The ions of the added electrolyte are adsorbed on the charged surface, reducing the surface potential of the particles. As a result of a decrease in charge, the electrical repulsive forces between particles weaken, and when particles approach each other, they stick together and precipitate.
Lowest electrolyte concentration WITH to, at which it begins slow coagulation is called coagulation threshold.
With a further increase in electrolyte concentration higher At the coagulation threshold, the coagulation rate first increases (section I in Figure 2) - this is an area of slow coagulation.
The region in which the coagulation rate ceases to depend on the electrolyte concentration is called the region of rapid coagulation (section II in Figure 2).
In electrolyte coagulation of the concentration type, the coagulation threshold Ck in accordance with the Deryagin-Landau rule is inversely proportional to the charge of counterions Z to the sixth power:
It follows from it that the values of the coagulation thresholds for single-, double- and triply charged ions are related as
The reciprocal of the coagulation threshold is called coagulating ability. The coagulating ability values for single-, double- and triple-charged counterions are related to each other as 1:64:729.
The coagulation threshold, kmol/m3, is calculated using the formula
Where WITH el - electrolyte concentration, kmol/m3;
V el, - the minimum volume of electrolyte that causes coagulation, m 3;
V sol - volume of sol, m3.
tion, while the molecular systems are determined
3. HETEROGENEITY OF COLLOIDAL SYSTEMS AS THE MAIN DIFFERENCE FROM MOLECULAR SOLUTIONS
We have already said that aggregative instability is a specific feature of colloidal systems. This property of colloidal systems is of great practical importance. It would not be an exaggeration to say that the main task of the technologist of a production process in which colloidal systems take place is either maintaining the aggregative stability of the system, or, conversely, ensuring known coagulation conditions.
Aggregative instability is the central problem of colloidal chemistry, and already at the beginning of the course it is necessary to consider, at least in the most general form, what reasons determine the aggregative instability of colloidal systems and why many colloidal systems, despite their fundamental aggregative instability, exist for a very long time. The reasons for the instability of colloidal systems can be explained from two points of view - thermodynamic and kinetic.
According to thermodynamics, the aggregative instability of colloidal systems is due to a sufficiently large and always positive free surface energy concentrated on the interphase surface of the system. Since surface energy represents free energy and since all systems with excess free energy are unstable, this determines the ability of colloidal systems to coagulate. During coagulation, particles stick together, and the interfacial surface at least partially disappears and, thus, the free energy of the system decreases. However, Smoluchowski, and more recently G. A. Martynov, drew attention to the fact that to reduce the free energy of a system, direct contact of particles is not necessary. Free energy can also decrease when the particles do not come into direct contact, but approach only at a certain distance, allowing them to interact through the layer separating their media.
In fact, let
where F is the free surface energy of the entire system; st, % - interfacial surface; f - specific free surface energy.
The quantity f is the sum of the interfacial surface energy fa, determined by the state of the monolayer at the phase boundary, and the free energy fv near the surface, i.e. f = fa+ fv. The volume-surface contribution fv is due to a change in the state of the liquid layers near the phase interface. Despite the fact that in general fa^fv, the stability of the system “in most cases is associated precisely with the change in fv, since during the formation of aggregates from solid particles the phase boundary usually does not disappear. Therefore, during coagulation, the value of /a remains practically constant, but fv changes , and the degree of change depends on the decrease in the distance between the particles. Of course, all this does not apply to emulsions where coalesceation takes place, that is, the merging of particles with the complete elimination of the interphase surface that originally separated the particles.
Since colloidal systems, which have a large specific surface area and high free energy, are fundamentally nonequilibrium systems, the well-known phase rule is not applicable to them. Such systems will obviously always tend to an equilibrium state corresponding to the division of the system into two continuous phases with a minimum interphase surface, although this equilibrium may practically never occur. The thermodynamic interpretation of the reasons for the stability or instability of colloidal systems is extremely simple. However, like any thermodynamic interpretation, this explanation is formal, i.e., it does not reveal the essence of the property of aggregative instability. In addition, thermodynamics does not establish a connection between the free energy of a system and how long the system can remain in a nonequilibrium state. Therefore, a more complete explanation in this case is the explanation of aggregative instability or stability of colloidal systems from the standpoint of physical kinetics.
According to kinetic concepts, the instability or stability of a colloidal or microheterogeneous system is determined by the ratio of forces acting between its individual particles. These forces include forces of two kinds: cohesive forces, or attraction forces, which tend to bring particles together and form an aggregate from them, and repulsive forces, which prevent coagulation.
Cohesion forces are usually of the same nature as intermolecular (van der Waals) forces. It is important that the forces acting between particles increase very quickly as the particles approach each other.
Repulsive forces can be electrical forces arising as a result of selective adsorption by the interfacial surface of one of the electrolyte ions present in the system. Since the particles of the dispersed phase are identical in nature and always adsorb a certain ion, they all acquire an electric charge of the same sign and experience mutual repulsion, which prevents them from approaching such distances where very significant attraction forces can already act. Another reason that prevents the convergence of colloidal particles to distances at which adhesion forces begin to prevail may be the formation of a solvation shell of medium molecules on the surface of the particles. Such a shell arises as a result of adsorption by the dispersed phase of either medium molecules or molecules or ions of the third component (stabilizer) of the system. In addition to these two factors, there are other factors that provide aggregate stability to colloidal systems. All sustainability factors are discussed in detail in Chapter. IX.
Thus, the relative stability of a colloidal system is determined by whether the repulsive forces are strong enough to prevent particles from approaching close distances. It is clear that such an explanation does not contradict the fundamental instability of the vast majority of colloidal systems, since when the surfaces of particles are in close proximity, the adhesion forces are, as a rule, greater than the repulsive forces and it is usually energetically more favorable for two separate particles to form an aggregate. We will see later that there are many ways to reduce repulsive forces, and in particular, one of these methods is the introduction of electrolytes into the system.
4. DISCOVERING PRESSURE*
* This section of the chapter was written by B.V. Deryagiy.
When the layer of liquid separating the surfaces of two solids or, in general, any two phases that have adsorbed ions becomes thinner, two types of interaction forces arise between the surfaces of these phases. First, the forces depending on the attraction between the molecules of both bodies, between the molecules of the liquid and between the molecules of the liquid and each body (or phase).
If both bodies are the same, then these forces lead to an attraction between the bodies, which tends to thin the layer of liquid. Secondly, as a result of the action of forces of an electrical nature, repulsion always occurs between identical bodies, causing a thickening of the liquid layer. Therefore, so that the thickness of the layer does not change and the system as a whole maintains its
As indicated in § 106, a qualitative feature of dispersed systems is their aggregative instability.
Prevention of aggregation of primary dispersed particles is possible as a result of the action of three factors of stability of dispersed systems: 1) kinetic; 2) electrical and 3) structural-mechanical.
A necessary condition for the adhesion of two particles of the dispersed phase is their approach, sufficient for the manifestation of attractive forces. If the frequency of collisions of colloidal particles is small, then the dispersed system can be stable (kinetic stability factor). This can occur at a very low concentration of dispersed particles (for example, in some aerosols) or at a very high viscosity of the dispersion medium (for example, in disperse systems of the T 1 -T 2 type).
Most stable disperse systems, in addition to the dispersed phase and dispersion medium, contain a third component, which is a dispersion stabilizer. The stabilizer can be both ions and molecules, and therefore two mechanisms for stabilizing dispersed systems are distinguished: electrical and molecular adsorption.
Electrical stabilization of dispersed systems associated with the appearance of a double electrical layer at the interface. Such stabilization is of primary importance for obtaining stable lyosols and suspensions in polar environments, such as water. In any hydrolysis, all colloidal particles have the same sign of charge. However, the colloidal micelle is generally electrically neutral as a result of the formation of an electrical double layer. Therefore, electrostatic repulsion between colloidal particles (. electrical stability factor) occurs only when they are sufficiently close, when their ionic atmospheres overlap (Fig. 102). The greater the overlap of the diffuse parts of the double electrical layer of colloidal particles, the greater the potential energy of electrostatic repulsion, i.e. the smaller the distance (x) between them and the greater the thickness of the electrical double layer.
Rice. 102.
In addition to electrostatic repulsion, between colloidal particles, as well as between molecules of any substance, there are intermolecular forces of attraction, among which dispersion forces play the largest role. The dispersion forces acting between individual molecules quickly decrease with increasing distance between them. But the interaction of colloidal particles is due to the summation of dispersion forces of attraction between all molecules located on the contact surface of colloidal particles. Therefore, the forces of attraction between colloidal particles decrease more slowly and occur over greater distances than in the case of individual molecules.
Potential energy of interaction (U) between colloidal particles is the algebraic sum of the potential energy of electrostatic repulsion (U 3) and potential energy of dispersion attraction (U a) between them:
![]()
If U 3 > U a(in absolute value), then repulsion prevails over attraction and the dispersed system is stable. If U 3 then the colloidal particles colliding during Brownian motion stick together into larger aggregates and sedimentation of the latter occurs. Colloidal solution coagulates, those. is divided into coagulate (sediment) and dispersion medium.
This is the essence of the theory of electrical stabilization and coagulation of dispersed systems, first developed by B.V. Deryagin (1937), and then L.D. Landau and Dutch scientists Verwey and Overbeck (1948); Based on the first letters of the authors' surnames, it is called the DLFO theory.

Rice. 103.
1 - electrical repulsion ( U 3); 2 - dispersion attraction (1/d): 3 - resultant interaction energy (JJ)] 4- the same, but with a steeper drop in curve 1] x - distance between particles; U m3kc - potential barrier to interaction of dispersed particles
In Fig. 103 shows the dependences of the quantities Ua And U 3 on the distance between colloidal particles. In this case, as is customary in physics, the potential energy of attraction is assigned a minus sign, and the potential energy of repulsion is assigned a plus sign. As can be seen, the resulting interaction energy (curve 3 in Fig. 103) leads to attraction (U(JJ > 0) at large distances between particles. The magnitude of the potential repulsive barrier is of decisive importance for the stability of dispersed systems. (U m3kc), which, in turn, depends on the course of the curves Ua And U 3 . At large values of this barrier, the colloidal system is stable. The adhesion of colloidal particles is possible only when they are sufficiently close. This requires overcoming the potential barrier of repulsion. For some small positive values U m3kc (curve 3) only a few colloidal particles with sufficiently high kinetic energy can overcome it. This corresponds to the stage of slow coagulation, when only a small part of the collisions of colloidal particles leads to their sticking together. With slow coagulation, over time there is a slight decrease in the total number of colloidal particles as a result of the formation of aggregates of 2-3 primary particles, but the coagulum does not precipitate. Such coagulation, not accompanied by a visible change in the colloidal solution, is called hidden coagulation. With a further decrease in the potential barrier, the coagulation rate, characterized by a change in the number of particles per unit time, increases. Finally, if the potential barrier passes from the repulsive region to the attractive region (curve 4 in Fig. 103), comes fast coagulation, when each collision of colloidal particles leads to their sticking together; in a colloidal solution a precipitate is formed - a coagulum, occurs obvious coagulation.
Potential repulsion barrier (U m1ikc) arises as a result of the summation of repulsive and attractive forces acting between colloidal particles. Therefore, all factors influencing the course of the curves 1 And 2 (Fig. 103), lead to a change in both the value U mskc , and the position of the maximum (i.e. the distance X, corresponding?/max).
Significant reduction U mskc occurs as a result of a change in the potential energy of electrostatic repulsion (i.e., the course of the curve 1), caused by the addition of electrolytes to a colloidal solution. With an increase in the concentration of any electrolyte, a restructuring of the electrical double layer surrounding the colloidal particles occurs: an increasing part of the counterions is displaced from the diffuse to the adsorption part of the electrical double layer. The thickness of the diffuse part of the double electrical layer (layer 4 in Fig. 100), and with it the entire double electrical layer (layer 2 in Fig. 100) decreases. Therefore, the potential energy curve of electrostatic repulsion decreases more steeply than that shown in Fig. 103 curve 1. As a result, a potential repulsive barrier (U mskc) decreases and shifts towards a smaller distance between colloidal particles. When the electric double layer is compressed to the thickness of the adsorption layer (layer 3 in Fig. 100), then the entire interaction curve of dispersed particles appears in the area of attraction (curve 4 in Fig. 103), rapid coagulation occurs. This change in the stability of a colloidal solution occurs when any electrolyte is added.
The coagulating effect of electrolytes is characterized by coagulation threshold, those. the lowest electrolyte concentration causing coagulation. Depending on the nature of the electrolyte and colloidal solution, the coagulation threshold varies from IO -5 to 0.1 mol per liter of sol. The most significant influence on the coagulation threshold is charge coagulating ion electrolyte, i.e. an ion whose charge is opposite in sign to the charge of the colloidal particle.
Multiply charged counterions of the electrolyte have an increased adsorption capacity compared to singly charged ones and penetrate into the adsorption part of the electrical double layer in large quantities. In this case, the coagulation threshold decreases not in proportion to the charge of the counterion, but much faster.
A brilliant confirmation of the DLFO theory was the calculation of B.V. Deryagin and L.D. Landau (1941) relationship between the threshold values of coagulation caused by electrolytes containing ions with different charge values. It turned out that the coagulation threshold is inversely proportional to the sixth power of the charge of the coagulating ion. Consequently, the values of the coagulation thresholds for single-, double-, triple- and quadruple-charged ions should be related as
which is close to the ratios of electrolyte concentrations that were observed during the coagulation of various hydrosols. This is illustrated by the data in Table. 22, which shows the equivalent concentrations of electrolytes (S to), causing coagulation of arsenic (III) oxide hydrosol.
Table 22
Coagulation thresholds (C k negatively charged sol As 2 O 3 electrolytes)
|
Electrolyte |
C k -IO 3 , n. |
Electrolyte |
C k -IO 3 , And. |
||
|
(C k)uci |
|||||
Molecular adsorption stabilization of disperse systems plays a major role in the stability of dispersions in both aqueous and non-aqueous media. Dispersed systems in non-aqueous media are, in principle, less stable than in an aquatic environment. In a non-polar and water-free dispersion medium, the particles of the dispersed phase are devoid of electrical charge. There is no electrical stabilization factor. Only forces of mutual attraction act between dispersed particles. The weakening of these forces, leading to the stabilization of dispersed systems, can occur as a result of the formation around colloidal particles of adsorption layers from molecules of the dispersion medium and substances dissolved in it. Such layers weaken the mutual attraction of particles of the dispersed phase and create a mechanical obstacle to their approach.
Stabilization of dispersed systems due to solvation of the dispersed phase by molecules of the dispersion medium is possible in both polar and non-polar media. Thus, the hydration of clay and silicic acid particles is essential for the stability of suspensions of clays and silicic acid sol in an aqueous environment.
However, the stabilization of dispersed systems is much more effective when surfactants and high-molecular compounds adsorbed at the phase interface are added to them. Adsorption layers of surfactants and high-molecular compounds, having elasticity and mechanical strength, prevent the sticking of dispersed particles. The formation of such molecular adsorption solid surface layers P.A. Rebinder called structural-mechanical factor for stabilizing dispersed systems. This stabilization mechanism plays a major role in obtaining extremely stable highly concentrated foams, emulsions, colloidal solutions and suspensions not only in non-aqueous but also in aqueous media. For structural and mechanical stabilization of dispersions in an aqueous environment, alkali metal soaps, proteins, and starch are used, and in non-aqueous media, alkaline earth metal soaps, resins, and rubbers are used. Such substances are called protective colloids.
- Boris Vladimirovich Deryagin (1902-1994) - academician, author of the modern theory of stability and coagulation of colloids, the electrical theory of gluing and adhesion, and important research in the field of aerosols.
- Pyotr Aleksandrovich Rebinder (1898-1972) - Soviet physicist-chemist, academician, State Prize laureate, founder of a large scientific school in the field of physical chemistry of dispersed systems. The ways he developed to control the properties of dispersed systems and the processes of their formation and destruction are closely related to the solution of major technical problems.
The textbook is intended for students of non-chemical specialties of higher educational institutions. It can serve as a guide for individuals independently studying the basics of chemistry, and for students of chemical technical schools and senior high schools.
A legendary textbook, translated into many languages of Europe, Asia, Africa and published in a total circulation of over 5 million copies.
When producing the file, the site http://alnam.ru/book_chem.php was used
Book:
| <<< Назад
|
Forward >>> |
As indicated in § 106, a qualitative feature of dispersed systems is their aggregative instability.
Prevention of aggregation of primary dispersed particles is possible as a result of the action of three factors of stability of dispersed systems: 1) kinetic, 2) electrical and 3) structural-mechanical.
A necessary condition for the adhesion of two particles of the dispersed phase is their approach, sufficient for the manifestation of attractive forces. If the frequency of collisions of colloidal particles is small, then the dispersed system can be stable (kinetic stability factor). This can occur at a very low concentration of dispersed particles (for example, in some aerosols) or at a very high viscosity of the dispersion medium (for example, in disperse systems of the T 1 -T 2 type).
Rice. 102. Scheme of overlapping ionic atmospheres of two colloidal particles.
Most stable disperse systems, in addition to the dispersed phase and dispersion medium, also contain a third component, which is a dispersion stabilizer. The stabilizer can be both ions and molecules, and therefore two mechanisms for stabilizing dispersed systems are distinguished: electrical and molecular adsorption (p. 324),
Electrical stabilization of disperse systems is associated with the appearance of a double electrical layer at the phase interface. Such stabilization is of primary importance for obtaining stable lyosols and suspensions in polar environments, such as water. In any hydrolysis, all colloidal particles have the same sign of charge. However, the colloidal micelle is generally electrically neutral as a result of the formation of an electrical double layer. Therefore, electrostatic repulsion between colloidal particles (electric stability factor) occurs only when they are sufficiently close, when their ionic atmospheres overlap (Fig. 102). The greater the overlap of the diffuse parts of the electrical double layer of colloidal particles, i.e., the smaller the distance (x) between them and the greater the thickness of the electrical double layer, the greater the potential energy of electrostatic repulsion.
In addition to electrostatic repulsion, between colloidal particles, as well as between molecules of any substance, there are intermolecular forces of attraction, among which dispersion forces play the largest role. The dispersion forces acting between individual molecules quickly decrease with increasing distance between them. But the interaction of colloidal particles is due to the summation of dispersion forces of attraction between all molecules located on the contact surface of colloidal particles. Therefore, the forces of attraction between colloidal particles decrease more slowly and occur over greater distances than in the case of individual molecules.
The potential energy of interaction (U) between colloidal particles is the algebraic sum of the potential energy of electrostatic repulsion (U e) and the potential energy of dispersion attraction (U d) between them:
If U e > U d (in absolute value), then repulsion prevails over attraction and the dispersed system is stable.

Rice. 103. Potential energy of interaction between two equally charged particles: 1 - electrical repulsion (U e) 2 - dispersion attraction (U d); 3 - resulting interaction energy (U); 4 - the same, but with a steeper drop in curve 1; x is the distance between particles; U max is the potential barrier to the interaction of dispersed particles.
If If U e< U д, то происходит слипание сталкивающихся при броуновском движении коллоидных частиц в более крупные агрегаты и седиментация последних. Коллоидный раствор коагулирует, т. е. разделяется на коагулят (осадок) и дисперсионную среду.
This is the essence of the theory of electrical stabilization and coagulation of dispersed systems, first developed by B.V. Deryagin (1937), and then by L.D. Landau and the Dutch scientists Verwey and Overbeck (1948); Based on the first letters of the authors' surnames, it is called the DLFO theory.
In Fig. Figure 103 shows the dependences of the values of U d and U e on the distance between colloidal particles. In this case, as is customary in physics, the potential energy of attraction is assigned a minus sign, and the potential energy of repulsion is assigned a plus sign. As can be seen, the resulting interaction energy (curve 3 in Fig. 103) leads to attraction (U<0) на очень малых и отталкиванию (U>0) at large distances between particles. Of decisive importance for the stability of dispersed systems is the value of the potential repulsive barrier U max, which, in turn, depends on the course of the U d and U e curves. At large values of this barrier, the colloidal system is stable. The adhesion of colloidal particles is possible only when they are sufficiently close. This requires overcoming the potential barrier of repulsion. At some small positive values of U max (curve 3), only a few colloidal particles with a sufficiently large kinetic energy can overcome it. This corresponds to the stage of slow coagulation, when only a small part of the collisions of colloidal particles leads to their sticking together. With slow coagulation, over time there is a slight decrease in the total number of colloidal particles as a result of the formation of aggregates from primary particles, but the coagulum does not precipitate. Such coagulation, which is not accompanied by a visible change in the colloidal solution, is called latent coagulation.
With a further decrease in the potential barrier, the coagulation rate, characterized by a change in the number of particles per unit time, increases. Finally, if the potential barrier passes from the region of repulsion to the region of attraction (curve 4 in Fig. 103), rapid coagulation occurs, when each collision of colloidal particles leads to their sticking together; In the colloidal solution, a precipitate is formed - a coagulum, and obvious coagulation occurs.
The potential repulsive barrier (U max) arises as a result of the summation of the repulsive and attractive forces acting between colloidal particles. Therefore, all factors influencing the course of curves 1 and 2 (Fig. 103) lead to a change in both the value of U max and the position of the maximum (i.e., the distance X corresponding to U max).
A significant decrease in Umax occurs as a result of a change in the potential energy of electrostatic repulsion (i.e., the course of curve 1) caused by the addition of electrolytes to the colloidal solution. With an increase in the concentration of any electrolyte, a restructuring of the double electrical layer surrounding the colloidal particles occurs: an increasing part of the counter-ions is forced out from the diffuse to the adsorption part of the double electrical layer. The thickness of the diffuse part of the double electrical layer (layer 4 in Fig. 100), and with it the entire double electrical layer (layer 2 in Fig. 100) decreases. Therefore, the potential energy curve of electrostatic repulsion decreases more steeply than that shown in Fig. 103 curve 1. As a result, the potential barrier of repulsion (U max) decreases and shifts towards a smaller distance between colloidal particles. When the electric double layer is compressed to the thickness of the adsorption layer (layer 8 in Fig. 100), then the entire interaction curve of dispersed particles appears in the area of attraction (curve 4 in Fig. 103), and rapid coagulation occurs. This change in the stability of a colloidal solution occurs when any electrolyte is added.
The coagulating effect of electrolytes is characterized by the coagulation threshold, i.e., the lowest concentration of electrolyte that causes coagulation. Depending on the nature of the electrolyte and colloidal solution, the coagulation threshold varies from 10 -5 to 0.1 mol per liter of sol. The most significant influence on the coagulation threshold is exerted by the charge of the coagulating ion of the electrolyte, i.e., an ion whose charge is opposite in sign to the charge of the colloidal particle.
Multiply charged counterions of the electrolyte have an increased adsorption capacity compared to singly charged ones and penetrate into the adsorption part of the electrical double layer in large quantities. In this case, the coagulation threshold decreases not in proportion to the charge of the counterion, but much faster.
A brilliant confirmation of the DLFO theory was the calculation by B.V. Deryagin and L.D. Landau (1941) of the ratio of the values of the coagulation thresholds caused by electrolytes containing ions with different charge values. It turned out that the coagulation threshold is inversely proportional to the sixth power of the charge of the coagulating ion. Consequently, the values of the coagulation thresholds for single-, double-, triple- and quadruple-charged ions should be related as
which is close to the ratios of electrolyte concentrations that were observed during the coagulation of various hydrosols. This is illustrated by the data in Table. 22, which shows the equivalent concentrations of electrolytes C to cause coagulation of arsenic(III) oxide hydrosol.
Table 22. Coagulation thresholds (C to) of a negatively charged sol As 2 O 3 with electrolytes

Molecular adsorption stabilization of disperse systems plays an important role in the stability of dispersions in both aqueous and non-aqueous media. Dispersed systems in non-aqueous media are, in principle, less stable than in an aquatic environment. In a non-polar and water-free dispersion medium, the particles of the dispersed phase are devoid of electrical charge. There is no electrical stabilization factor. Only forces of mutual attraction act between dispersed particles. The weakening of these forces, leading to the stabilization of dispersed systems, can occur as a result of the formation around colloidal particles of adsorption layers from molecules of the dispersion medium and substances dissolved in it. Such layers weaken the mutual attraction of particles of the dispersed phase and create a mechanical obstacle to their approach.
Stabilization of dispersed systems due to solvation of the dispersed phase by molecules of the dispersion medium is possible in both polar and non-polar media. Thus, the hydration of clay and silicic acid particles is essential for the stability of suspensions of clays and silicic acid sol in an aqueous environment.
However, the stabilization of dispersed systems is much more effective when surfactants and high-molecular compounds adsorbed at the phase interface are added to them. Adsorption layers of surfactants and high-molecular compounds, having elasticity and mechanical strength, prevent the sticking of dispersed particles. P. A. Rebinder called the formation of such molecular adsorption solid surface layers a structural-mechanical factor in the stabilization of dispersed systems. This stabilization mechanism plays a major role in obtaining extremely stable highly concentrated foams, emulsions, colloidal solutions and suspensions not only in non-aqueous but also in aqueous media. For structural and mechanical stabilization of dispersions in an aqueous environment, alkali metal soaps, proteins, and starch are used, and in non-aqueous media, alkaline earth metal soaps, resins, and rubbers are used. Such substances are called protective colloids.
| <<< Назад
|
Forward >>> |
The modern physical theory of coagulation by electrolytes is based on the general principles of statistical physics, the theory of molecular forces and the theory of solutions. Its authors are: B.V. Deryagin, L.D. Landau (1937-1941), E. Verwey, J. Overbeck (according to the first letters DLFO).
The essence of the theory: Between any particles, when they come together, a disjoining pressure of the separating liquid layer arises as a result of the action of forces of attraction and repulsion. Disjoining pressure is a summary parameter that takes into account the action of both attractive and repulsive forces.
The state of the system depends on the balance of the energy of attraction (U pr) and the energy of repulsion (U ret). Prevails Uott - a stable system. Prevails U pr - violation of aggregative stability - coagulation.
The change in interaction energy between two particles as they approach each other is depicted graphically (Fig. 5.3).
The total energy of a system of two particles (curve 3) is obtained by adding U outt and U in:
U=U ott +U pr =
where: B is a multiplier that depends on the values of the electrical potentials of the diesel power plant, the properties of the environment, and temperature;
e – the base of the natural logarithm;
c is the reciprocal of the thickness of the diffuse layer;
h – distance between particles;
A is the constant of molecular attractive forces.
|
|||||
|
|||||
|
|||||
Fig.5.3. Potential interaction curves
colloidal particles:
1 – change in repulsion energy with distance;
2 – change in attraction energy;
3 – resulting curve.
Consider the resulting curve 3 in Fig. 5.3. It has characteristic areas:
In the region of small distances there is a deep primary minimum (potential well) - U ave significantly predominates. The primary minimum corresponds to the direct adhesion of particles (I).
In the region of large distances there is a secondary shallow minimum (the second potential well, which corresponds to attraction through a layer of the medium). In diagram II.
In the region of average distances, there is a maximum on the curve and, if it is located above the x-axis, then an energy barrier to repulsive forces (DU b) appears.
The resulting curve 3 may have a different appearance depending on the stability of the dispersed system (Fig. 5.4.).
|
|||
|
|||
Rice. 5.4. Potential curves for certain
states of stability of a dispersed system:
1 - in the system, at any distance between particles, the energy of attraction prevails over the energy of repulsion. In such a system, rapid coagulation with the formation of aggregates is observed.
2 - a fairly high potential barrier and the presence of a secondary minimum. Particles interact, but do not have direct contact and are separated by layers of the medium.
3 - a system with high aggregate stability (high potential barrier and the absence of a secondary minimum or, at its depth, less than the thermal energy kT).
Depending on the height of the energy barrier and the depth of potential wells, various options for the behavior of particles when approaching are possible (Fig. 5.5), the particles have kinetic energy - kT.
Fig.5.5. Schemes of interaction of colloidal particles
| State c: Low barrier height and shallow secondary minimum: DU b @DU I £kT particles enter into short-range interaction, i.e. come into direct contact - coagulation occurs | State a: Characterized by the fact that the diffuse layers overlap and the layers of the medium between the particles (gels) are preserved. The energy barrier is quite high; the secondary minimum is shallow: DU I ³kT Interacting particles cannot move apart (they are held back by attractive forces) and cannot approach closely (they are prevented by repulsive forces). Addition of an electrolyte most often leads to coagulation (h decreases). | State b: High energy barrier DU b ³kT and absence or shallow secondary minimum DU i £kT: Particles cannot overcome the barrier and disperse without interaction. Such a system is aggregatively stable. |
The dispersed system is aggregatively stable at a high energy barrier of repulsive forces.