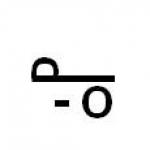Independent work on organic chemistry on the topics:
"Alkanes", "Alkenes", "Alkynes".
Chemistry teacher Zhuravleva T.K.
Independent work on the topic: Alkanes
Option #1
1. Match:
Concept:
1) homologues, 2) isomers.
Definition:
a) substances similar in structure and properties, the composition of which differs by one or more CH 2 groups.
b) substances that have the same composition, but different chemical structures.
2. General formula of alkanes
a) C n H 2 n -6, b) C n H 2 n -2, c) C n H 2 n, d) C n H 2 n +2.
3.
a) C 2 H 8, b) C 2 H 6, c) C 5 H 8, d) C 6 H 6.
Name this substance and write its full structural formula.
4. Find the formula of the pentane isomer:
a) CH 3 -CH-CH 3, b) CH 3 -CH 2, c) CH 2 -CH 2 d) CH 3 -CH 2
│ │ │ │ │
CH 3 CH 3 CH 3 CH 3 CH-CH 3
5. Homologues are substances:
a) C 3 H 8 and C 2 H 2, b) C 5 H 12 and C 3 H 6, c) C 2 H 2 and C 6 H 6, d) CH 4 and C 2 H 6
6. Alkanes are characterized by the following reactions:
a) addition b) substitution c) polymerization.
7. Match:
Alkane formula :
1) CH 3 -CH 2 -CH 2 -CH 2 2) CH 3 -CH 2 -CH 2 -CH 2
CH 3 CH 3 CH 2 - CH 3
Name:
a) 2-methylpentane, b) hexane
Option No. 2
1. Fill in the missing word:
a) Substances that are similar in structure and properties, but whose composition differs by one or more CH 2 groups, are called -___. b) Substances that have the same elemental composition but different chemical structures are called _________.
2. Saturated hydrocarbons have the general formula:
a) C n H 2 n -6, b) C n H 2 n -2, c) C n H 2 n, d) C n H 2 n +2
3. Specify the formula of the saturated hydrocarbon:
a) C 2 H 4, b) C 3 H 4, c) C 4 H 10, d) C 6 H 6.
4. Find the formula for the homologue of butane:
a) CH 3 -CH 2, b) CH 3 -CH 2 -CH-CH 3, c) CH 3 -CH 2 -CH 2 -CH 3
CH 2 -CH 2 -CH 3 CH 3
d) CH 3 -CH -CH 3
5. Homologues are substances of normal structure:
a) CH 4 and C 2 H 4, b) C 3 H 8 and C 5 H 12, c) C 4 H 8 and C 8 H 1 8, d) CH 4 and C 6 H 10
6. Make up the formulas of the isomer and homologue of hexane
7. Match:
Alkane formula: 1) CH 3 -CH 2 -CH-CH 2 CH 2 -CH 3 2) CH 3 -CH 2 - CH-CH-CH 3
C 2 H 5 CH 3 CH 3
Name:
a) 3-ethylhexane, b) 2,3-dimethylpentane
Independent work on the topic: Alkenes.
Option #1
a) homologue; b) isomer;
CH 2 = CH – CH – CH 2 – CH 2 - CH 3
CH 3
CH 3 |
a) CH 3 – CH = C – CH – CH 3 b) CH 2 = C – CH 2 – CH – CH 3
׀ ׀ ׀
CH 3 CH 3 CH 2 – CH 3
3.Obtain an alkene by dehydrogenation of butane.
4.Write the equations of chemical reactions and indicate the type of reactions:
a) CH 2 = CH – CH 3 + H 2 →
b) CH 3 - CH =CH – CH 3 + Br 2 →
c) C 2 H 4 + O 2 →
d) CH 2 = CH -CH 2 + HCl →
Option No. 2
1. Make up structural formulas:
a) homologue; b) isomer;
c) isomer of the position of the double bond.
for a substance having the structure
CH 3 – CH = CH – CH – CH 3
C2H5
2.Name the following hydrocarbons using systematic nomenclature:
CH 3
a) CH 2 = C – CH 2 – CH 2 b) CH 3 – CH= C – C – CH 3
׀ ׀ ׀
CH 3 CH 3 CH 3
3.Obtain propene by dehydration of alcohol.
4.Write the equations of chemical reactions and indicate the type of reaction:
a) CH 2 = CH – CH 2 – CH 3 + H 2 O →
b) CH 2 = CH 2 + H 2 →
c) CH 2 = CH 2 + HCL →
d) C 3 H 6 + O 2 →
Independent work
Alkene problems
The chemical properties are quite different from the properties of the double bond causing addition reactions; in general, alkenes are more reactive compounds. The general formula of the compounds is СnH2n.
In this problem on alkenes an oxidation reaction is given. In a solution of potassium permanganate, KMnO4 is oxidized to diols - dihydric alcohols, and the permanganate is reduced to manganese (IV) oxide. The reaction will look like this:
3|СnH2n + 2OH(-) -2e → CnH2n(OH)2
2 |MnO4(-) +2H2O +3e → MnO2 + 4OH(-)
3CnH2n +6OH(-) +2MnO4(-) + 4H2O → 3CnH2n(OH)2 + 2MnO2 + 8OH(-).
Final equation:
ZS n H 2 n + 2KMnO 4 + 4H 2 O → 3C n H 2 n (OH) 2 + 2MnO 2 ↓ + 2KOH
The precipitate formed is manganese (IV) oxide - MnO2. Let us denote the mass of the alkene as X. Then the mass of MnO2 will be equal to 2.07X
According to the reaction, alkene and manganese oxide react 3:2.
This means that the ratio of moles of substances can be written as follows:
X\ 3·(12n +2n) = 2.07x\87·2
where 12n+2n is the molar mass, and 87 g\mol is the molar mass of MnO2
n= 2
Those. alkene, which was subjected to oxidation - ethylene - C2H4.
Problem on alkenes No. 2

Chemical properties are determined by their ability to attach substances; a double bond turns into a single bond:
СnH2n + Cl2 → CnH2nCl2
CnH2n + Br2 → CnH2nBr2
m(CnH2nCl2) = 56.5 g\mol
m(CnH2nBr2) = 101 g\mol
The mass of the alkene that entered into the reaction is the same, which means the same number of moles.
Therefore, we express the number of moles of hydrocarbon - n(CnH2n):
m(CnH2nCl2) \ (12n+2n+71) = m(nH2nBr2) \ (12n+2n+160)
12n+2n+71 is the molar mass of the dichloro derivative,
(12n+2n+160) is the molar mass of the dibromo derivative.
56.5 \ (12n+2n+71) = 101 \ (12n+2n+160)
n= 3, alkene - C3H6
In contrast, they mainly undergo addition reactions. IN problems on alkenes all reactions are simple and usually boil down to determining the formula of the substance.
Problem on alkenes No. 3
Reaction equation:
Alkenes only react with a catalyst, so there will only be one reaction in this problem.
m(Br2) = m(solution) ω = 100 g 0.181 = 18.1 g
Let us denote the mass of bromine that reacted as x
Bromine in solution will be determined by the mass of unreacted bromine
m(Br2) = 18.1 - x.
Mass of solution = 100 + m(C3H6).
Unsaturated hydrocarbons.
Alkenes
GRADE 10
This lesson is a lesson in learning new material in the form of a lecture with elements of conversation and independent work of students.
Students work in three groups. In each group there is a teacher assistant who distributes work to each student in this group. Each student has a reminder.
REMINDER
Planned learning outcomesKnow: determination of unsaturated hydrocarbons of the ethylene series, the general formula of alkenes, four types of isomerism of alkenes, their physical and chemical properties, methods of production and areas of application of hydrocarbons of the ethylene series.
Be able to: explain the features of the formation of - and - bonds, write down the molecular, structural and electronic formulas of alkenes, designate the distribution of electron density in the molecule, name the substances of the ethylene series according to systematic nomenclature and write down their formulas using the names of the substances, create formulas for various isomers using the molecular formula of the alkene, write down equations reactions characterizing the chemical properties of alkenes, compare the properties of alkenes with the properties of saturated hydrocarbons, solve problems of finding the molecular formula.
Goals. Educational: learn to deduce the general formula of alkenes, know their physical and chemical properties, be able to write down the molecular and structural formulas of alkenes, name substances according to systematic nomenclature, develop skills in solving problems to find the molecular formula.
Educational: cultivate a desire to learn actively, with interest, instill conscious discipline, clarity and organization in work, work under the motto: “One for all, and all for one.”
Lesson methods and techniques
- Individual work with cards.
- Work in groups and pairs.
- Demonstration chemical experiment.
- Use of technical teaching aids.
- Independent work on drawing up formulas of substances.
- Oral answers at the board.
- Taking notes from the textbook in a notebook.
Lesson topic plan
(written on the board)
1. The structure of the ethylene molecule C 2 H 4.
2. Isomerism and nomenclature of alkenes.
3. Preparation of alkenes.
4. Physical properties.
5. Chemical properties.
6. Application.
7. Genetic connection.
Equipment and reagents. Cards with tasks, a graphic projector and slides, a tripod, a device for obtaining and collecting gases, an alcohol lamp, test tubes, sand, a chemical spoon; ethyl alcohol, potassium permanganate, bromine water, sulfuric acid (conc.).
DURING THE CLASSES
The lesson begins with a conversation in the form frontal poll. The purpose of this part of the lesson is to create a “success situation.” Students understand the questions, they know the answers to them and are actively involved in the work.
1. What is the bond length?
(The bond length is the distance between the centers
nuclei of bonded atoms in a molecule.)
2. What can be said about the carbon-carbon bond length of substances with a single (C–C) and double (C=C) bond?
(Length of carbon-carbon single bond – 0.154 nm
double bond – 0.133 nm, double bond is stronger and shorter than single bond.)
3. How many -bonds can arise between atoms?
4. What can be said about the strength of the bond?
(It is less durable than single - connection.)
5. What chemical bond is formed between hybridized clouds?
6. How many valence electrons does a carbon atom have?
Independent work.
Derivation of molecular formula
Task. In the compound, the mass fraction of carbon is 85.7%, the mass fraction of hydrogen is 14.3%, the density of hydrogen is 14. Derive the molecular formula of the hydrocarbon.
(One of the students decides at the board.)
Given:
(C) = 85.7% (or 0.857),
(H) = 14.3% (or 0.143),
D(H2) = 14.
Find:
C x H y .
Solution
M(C x H y) = 14 2 = 28 g/mol.
For 1 mol C x H y m(C x H y) = 28 g,
m(C) = 28 (g) 0.857 = 24 g,
n(C) = 24 (g)/12 (g/mol) = 2 mol,
m(H) = 28 (g) 0.143 = 4 g,
n(H) = 4 (g)/1 (g/mol) = 4 mol.
The hydrocarbon formula is C 2 H 4.
We conclude that the C 2 H 4 molecule is not saturated with hydrogen atoms.
The structure of the ethylene molecule C 2 H 4
We demonstrate a model of a given hydrocarbon molecule through a graphic projector.
The C 2 H 4 molecule is flat, the carbon atoms forming the double bond are in the state
sp 2-hybridization, bond angle 120°.
We compose a homological series: C 2 H 4, C 3 H 6, C 4 H 8 ... and derive the general formula C n H 2 n .
Let's sum up the stage we've passed.
Isomerism and nomenclature of alkenes
Types of isomerism
1) Consider the structural formulas of linear and branched alkenes having the same molecular formula C 4 H 8:

This type of isomerism is called carbon skeleton isomerism.
2) Isomerism of multiple bond position:
3) Isomerism of different homologous series. General formula C n H 2 n corresponds to two homologous series: alkenes and cycloparaffins. For example, the formula C 4 H 8 may belong to compounds of different classes:

4) Spatial or geometric isomerism. In butene-2 CH 3 – CH = CH – CH 3, each carbon at the double bond has different substituents (H and CH 3). In such cases, cistrans isomerism is possible for alkenes. If the elements of the main carbon chain are on one side of the double bond in the plane of the molecule, then this is cisisomer; if on opposite sides, then this trans isomer:

Independent work using cards (5 min)
Name the substances.
1st group:

2nd group:

3rd group:

The completed independent work is recorded on film and projected through a graphic projector onto the screen. Students exercise self-control.
Preparation of alkenes
1) Dehydration of alcohols (demonstration experience of producing ethylene from ethyl alcohol):
![]()
2) Dehydrogenation of alkanes:

3) Pyrolysis and cracking of oil and natural gas:
4) From halogenated alkanes:

Physical properties
Alkenes - ethene, propene and butene - under normal conditions (20 ° C, 1 atm) - gases, from C 5 H 10 to C 18 H 36 - liquids, higher alkenes - solids. Alkenes are insoluble in water, but soluble in organic solvents.
Chemical properties
In organic chemistry, three types of chemical reactions are considered: substitution, addition and decomposition.
1) Alkenes are characterized by addition reactions.
Addition of hydrogen (hydrogenation):
![]()
Addition of halogens (laboratory experiment on decolorization of bromine water):
![]()
Addition of hydrogen halides:
![]()
Markovnikov's rule: hydrogen attaches at the site of a multiple bond to a more hydrogenated carbon, and a halogen to a less hydrogenated one.
For example:
The reaction proceeds by an ionic mechanism.
Addition of water (hydration reaction):
![]()
2) Oxidation reactions.

Demonstration experience. Ethene discolors a solution of potassium permanganate, which proves the unsaturated nature of ethene:
Ethylene glycol is used as an antifreeze; it is used to make lavsan fiber and explosives.
Oxidation of ethene on a silver catalyst produces ethylene oxide:

Ethylene oxide is used to produce acetaldehyde, detergents, varnishes, plastics, rubbers and fibers, and cosmetics.
3) Polymerization reaction.
The process of combining many identical molecules into larger ones is called a polymerization reaction.
 Determine the molecular formula of a hydrocarbon that contains 85.7% carbon and has a hydrogen density of 21.
Determine the molecular formula of a hydrocarbon that contains 85.7% carbon and has a hydrogen density of 21.
Given:
(C) = 0.857 (or 85.7%),
D(H2) = 21.
Find:
Solution
M(C x H y) = D(H2) M(H 2) = 21 2 = 42 g/mol.
For n(C x H y) = 1 mol m(C) = 42 0.857 = 36 g,
n(C) = 36 (g)/12 (g/mol) = 3 mol,
m(H) = 42 – 36 = 6 g,
n(H) = 6 (g)/1 (g/mol) = 6 mol.
The hydrocarbon formula is C 3 H 6 (propene).
Task 3.When 4.2 g of a substance is burned, 13.2 g of carbon monoxide (IV) and 5.4 g of water are formed. The vapor density of this substance in air is 2.9. Determine the composition of the hydrocarbon molecule.
Given:
m(C x H y) = 4.2 g,
m(CO 2) = 13.2 g,
m(H 2 O) = 5.4 g,
D(air) = 2.9.
Find: C x H y .
Solution
M(C x H y) = 2.9 29 = 84 g/mol.
To solve the problem, let's create a reaction equation:
![]()
Let's find the mass X mole of CO 2 and the corresponding amount of substance:
m(CO 2) = 84 13.2/4.2 = 264 g,
n(CO 2) = 264 (g)/44 (g/mol) = 6 mol, X = 6.
Likewise m(H 2 O) = 84 5.4/4.2 = 108 g,
n(H 2 O) = 108 (g)/18 (g/mol) = 6 mol, y = 12.
C 6 H 12 – hexene.
Each group submits the tasks they completed on pieces of paper. This is followed by a summary of the lesson.
Homework.Rudzitis G.E., Feldman F.G. Chemistry-10. M.: Education, 1999, chapter IV, § 1, p. 30–38, fig. 10, p. 38. Prepare questions 6, 7 from the plan for studying the lesson topic for the seminar, learn the material of the lesson-lecture.
Independent work
on this topic:
I option
1. Saturated hydrocarbons are characterized by the following reactions:
a) combustion, b) substitution, c) addition. d) neutralization?
2. A chain of transformations is given
1 2 3
C 2 H 6 → C 2 H 5 Cl→ C 4 H 10 → CO 2:
the second reaction is called a) Konovalov, b) Wurtz, c) Semenov.
Write down equations for all reactions.
3.
What compound is used to produce methane in the laboratory:
a) CH3COOH, b) CH 3 OH, c) CH 3 C1, d) C H 3 SOO N A
Write an equation for this reaction.
4.
The mass fractions of carbon and hydrogen in a hydrocarbon are equal, respectively
82.76% and 17.24%.
Its vapor density for hydrogen is 29. Derive the formula of the substance. Numberhydrogen atoms in a molecule are a) 12; b) 6; c) 10 d) 14.
"Chemical properties and methods of obtaining alkanes"
II option
1. Which of the following chemical properties are characteristic of methane:
a) hydrogenation, b) isomerization, c) combustion, d) catalytic oxidation?
Write down the corresponding reaction equations.
2. As a result of the following transformations
Cl 2 hlNa
CH 4 → X 1 → X 2
the final product is formed (X 2 )
a) propane, b) chloroethane, c) ethane, d) chloromethane?
Write the equations for all reactions.
3.
Indicate which compound is used to produce ethane (according to the reaction
Wurtz): a) C 2 H 4, b) CH 3 I, c) CH 3 - O - CH 3, d) C 2 H 5 OH?
4.
The mass fraction of carbon in the alkane is 81.82%, hydrogen 18.18%. Relative
its vapor density in air is 1.518. Determine the formula of the alkane. The number of carbon atoms in an alkane molecule is a) 4; b) 2; at 6; d) 3.
Give two homologues and two isomers for this hydrocarbon and give them names.
Independent work on the topic:
"Chemical properties and methods of obtaining alkanes"
III option
1. Indicate which of the following reactions are characteristic of butane:
a) addition, b) cracking, c) isomerization, d) dehydrogenation.
Write down equations for these reactions.
2.
What reaction can be used to obtain methane in the laboratory:
a) CH 3 OH + H 2 → b) CH 3 Br + N a →
c) CaC 2 + H 2 0 → g ) A1 4 C 3 + H 2 0 →
Write an equation for the corresponding reaction.
3. Indicate the conditions that are necessary for the start of the reaction between ethane and chlorine: a) cooling, b) heating, c) increasing pressure, d) lighting. Write an equation for the corresponding reaction
4. The mass fractions of carbon and hydrogen in the hydrocarbon are 81.8% and 18.2%, respectively. Its vapor density for hydrogen is 22.
The number of hydrogen atoms in a molecule of a substance is a) 8; b) 6; at 3; d)12.
Give two homologues and two isomers for this hydrocarbon and give them names.
Independent work