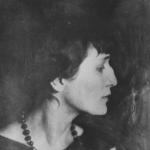The periodic system of Dmitry Ivanovich Mendeleev and its significance for natural science
Introduction
D.I. Mendeleev's discovery of patterns in the structure of matter turned out to be a very important milestone in the development of world science and thought. The hypothesis that all substances in the Universe consist of only a few dozen chemical elements seemed completely incredible in the 19th century, but it was proven by Mendeleev’s “Periodic Table of Elements.”
The discovery of the periodic law and the development of the periodic system of chemical elements by D. I. Mendeleev were the pinnacle of the development of chemistry in the 19th century. A vast amount of knowledge about the properties of 63 elements known at that time was brought into order.
Periodic table of elements
D.I. Mendeleev believed that the main characteristic of elements is their atomic weights, and in 1869 he first formulated the periodic law.
The properties of simple bodies, as well as the forms and properties of compounds of elements, are periodically dependent on the atomic weights of the elements.
Mendeleev divided the entire series of elements, arranged in order of increasing atomic masses, into periods, within which the properties of the elements change sequentially, placing the periods so as to highlight similar elements.
However, despite the enormous significance of such a conclusion, the periodic law and Mendeleev’s system represented only a brilliant generalization of facts, and their physical meaning remained unclear for a long time. Only as a result of the development of physics of the 20th century - the discovery of the electron, radioactivity, the development of the theory of atomic structure - the young, talented English physicist G. Mosle established that the magnitude of the charges of atomic nuclei consistently increases from element to element by one. With this discovery, Mosle confirmed the brilliant guess of Mendeleev, who in three places of the periodic table moved away from the increasing sequence of atomic weights.
Thus, when compiling it, Mendeleev placed 27 Co in front of 28 Ni, 52 Ti in front of 5 J, 18 Ar in front of 19 K, despite the fact that this contradicted the formulation of the periodic law, that is, the arrangement of elements in order of increasing atomic weights.
According to Mosle's law, the charges of nuclei of these elements corresponded to their position in the table.
In connection with the discovery of Mosle's law, the modern formulation of the periodic law is as follows:
the properties of elements, as well as the forms and properties of their compounds, are periodically dependent on the charge of the nucleus of their atoms.
So, the main characteristic of an atom is not the atomic mass, but the magnitude of the positive charge of the nucleus. This is a more general accurate characteristic of an atom, and therefore an element. All properties of the Element and its position in the periodic table depend on the magnitude of the positive charge of the atomic nucleus. Thus, The serial number of a chemical element numerically coincides with the charge of the nucleus of its atom. The periodic table of elements is a graphic representation of the periodic law and reflects the structure of the atoms of the elements.
The theory of atomic structure explains the periodic changes in the properties of elements. An increase in the positive charge of atomic nuclei from 1 to 110 leads to a periodic repetition of the structural elements of the external energy level in atoms. And since the properties of elements mainly depend on the number of electrons at the outer level; then they repeat periodically. This is the physical meaning of the periodic law.
As an example, consider the change in properties of the first and last elements of periods. Each period in the periodic system begins with elements of atoms, which at the outer level have one s-electron (incomplete outer levels) and therefore exhibit similar properties - they easily give up valence electrons, which determines their metallic character. These are alkali metals - Li, Na, K, Rb, Cs.
The period ends with elements whose atoms at the outer level contain 2 (s 2) electrons (in the first period) or 8 (s 1 p 6) electrons (in all subsequent ones), that is, they have a completed external level. These are noble gases He, Ne, Ar, Kr, Xe, which have inert properties.
It is precisely because of the similarity in the structure of the external energy level that their physical and chemical properties are similar.
In each period, with an increase in the ordinal number of the elements, the metallic properties gradually weaken and non-metallic ones increase, and the period ends with an inert gas. In each period, with an increase in the ordinal number of the elements, the metallic properties gradually weaken and non-metallic ones increase, and the period ends with an inert gas.
In the light of the doctrine of the structure of the atom, the division of all elements into seven periods made by D. I. Mendeleev becomes clear. The period number corresponds to the number of energy levels of the atom, that is, the position of elements in the periodic table is determined by the structure of their atoms. Depending on which sublevel is filled with electrons, all elements are divided into four types.
1. s-elements. The s-sublayer of the outer layer (s 1 - s 2) is filled. This includes the first two elements of each period.
2. p-elements. The p-sublevel of the external level is filled (p 1 -- p 6) - This includes the last six elements of each period, starting from the second.
3. d-elements. The d-sublevel of the last level (d1 - d 10) is filled, and 1 or 2 electrons remain at the last (outer) level. These include elements of plug-in decades (10) of large periods, starting from the 4th, located between the s- and p-elements (they are also called transition elements).
4. f-elements. The f-sublevel of the deep (one third of it outside) level is filled (f 1 -f 14), and the structure of the external electronic level remains unchanged. These are lanthanides and actinides, located in the sixth and seventh periods.
Thus, the number of elements in periods (2-8-18-32) corresponds to the maximum possible number of electrons at the corresponding energy levels: in the first - two, in the second - eight, in the third - eighteen, and in the fourth - thirty-two electrons. The division of groups into subgroups (main and secondary) is based on the difference in the filling of energy levels with electrons. The main subgroup consists s- and p-elements, and a secondary subgroup - d-elements. Each group combines elements whose atoms have a similar structure of the external energy level. In this case, the atoms of the elements of the main subgroups contain at the outer (last) levels a number of electrons equal to the group number. These are the so-called valence electrons.
For elements of side subgroups, the valence electrons are not only the outer ones, but also the penultimate (second outer) levels, which is the main difference in the properties of the elements of the main and side subgroups.
It follows that the group number usually indicates the number of electrons that can participate in the formation of chemical bonds. This is physical meaning of the group number.
From the standpoint of the theory of atomic structure, the increase in the metallic properties of elements in each group with increasing charge of the atomic nucleus is easily explained. Comparing, for example, the distribution of electrons by levels in atoms 9 F (1s 2 2s 2 2р 5) and 53J (1s 2 2s 2 2р 6 3s 2 Зр 6 3d 10 4s 2 4 R 6 4 d 10 5s 2 5p 5) it can be noted that they have 7 electrons in the outer level, which indicates similar properties. However, the outer electrons in an iodine atom are further away from the nucleus and are therefore less tightly held. For this reason, iodine atoms can donate electrons or, in other words, exhibit metallic properties, which is not typical for fluorine.
So, the structure of atoms determines two patterns:
a) change in the properties of elements horizontally - in a period, from left to right, metallic properties are weakened and non-metallic properties are enhanced;
b) change in the properties of elements vertically - in a group, with increasing serial number, metallic properties increase and non-metallic properties weaken.
Thus: As the charge of the nucleus of atoms of chemical elements increases, the structure of their electronic shells periodically changes, which is the reason for the periodic change in their properties.
The structure of the periodic system of D. I. Mendeleev.
The periodic system of D.I. Mendeleev is divided into seven periods - horizontal sequences of elements arranged in increasing order of atomic number, and eight groups - sequences of elements with the same type of electronic configuration of atoms and similar chemical properties.
The first three periods are called small, the rest - large. The first period includes two elements, the second and third periods - eight each, the fourth and fifth - eighteen each, the sixth - thirty-two, the seventh (incomplete) - twenty-one elements.
Each period (except the first) begins with an alkali metal and ends with a noble gas.
Elements of periods 2 and 3 are called typical.
Small periods consist of one row, large ones - of two rows: even (upper) and odd (lower). Metals are located in even rows of large periods, and the properties of the elements change slightly from left to right. In odd rows of large periods, the properties of the elements change from left to right, as in the elements of periods 2 and 3.
In the periodic system, for each element its symbol and serial number, the name of the element and its relative atomic mass are indicated. The coordinates of the element's position in the system are the period number and the group number.
Elements with serial numbers 58-71, called lanthanides, and elements with numbers 90-103 - actinides - are placed separately at the bottom of the table.
Groups of elements, designated by Roman numerals, are divided into main and secondary subgroups. The main subgroups contain 5 elements (or more). The secondary subgroups include elements of periods starting from the fourth.
The chemical properties of elements are determined by the structure of their atom, or rather the structure of the electron shell of the atoms. Comparison of the structure of electronic shells with the position of elements in the periodic table allows us to establish a number of important patterns:
1. The period number is equal to the total number of energy levels filled with electrons in the atoms of a given element.
2. In small periods and odd series of large periods, as the positive charge of the nuclei increases, the number of electrons in the external energy level increases. This is associated with the weakening of metallic and strengthening of non-metallic properties of elements from left to right.
The group number indicates the number of electrons that can participate in the formation of chemical bonds (valence electrons).
In subgroups, as the positive charge of the nuclei of elemental atoms increases, their metallic properties become stronger and their non-metallic properties weaken.
History of the creation of the Periodic Table
Dmitry Ivanovich Mendeleev wrote in October 1897 in the article “Periodic Law of Chemical Elements”:
- After Lavoisier's discoveries, the concept of chemical elements and simple bodies was so strengthened that their study formed the basis of all chemical concepts, and as a result entered into all natural science. We had to admit that all substances accessible to research contain a very limited number of materially heterogeneous elements that do not transform into each other and have an independent, weighty essence, and that the entire diversity of natural substances is determined only by the combination of these few elements and the difference either in themselves or in their relative quantities , or if the quality and quantity of elements are the same - by the difference in their relative position, ratio or distribution. In this case, substances containing only one element should be called “simple” bodies, “complex” - two or more. But for a given element, there may be many modifications of simple bodies corresponding to it, depending on the distribution (“structure”) of its parts or atoms, i.e. from that type of isomerism called “allotropy”. So carbon, as an element, appears in the state of coal, graphite and diamond, which (taken in their pure form) produce the same carbon dioxide and in the same quantity when burned. For the “elements” themselves, nothing like this is known. They do not undergo modifications or mutual transformations and, according to modern views, represent the unchanging essence of a changing (chemically, physically and mechanically) substance, which is included in both simple and complex bodies.
The very widespread idea, in ancient times and to this day, of a “single or primary” matter, from which all the variety of substances is composed, has not been confirmed by experience, and all attempts aimed at this have turned out to refute it. Alchemists believed in the transformation of metals into each other, they proved this in various ways, but when verified, everything turned out to be either a deception (especially in relation to the production of gold from other metals), or an error and incompleteness of experimental research. However, one cannot help but notice that if tomorrow it turns out that metal A is transformed in whole or in part into another metal B, then it will not at all follow from this that simple bodies are capable of transforming into each other in general, as, for example, from the fact that that for a long time uranium oxide was considered a simple body, but it turned out to contain oxygen and actual metallic uranium - no general conclusion should be made at all, but one can only judge in particular the former and modern degrees of familiarity with uranium as an independent element. From this point of view, we should also look at the transformation of Mexican silver partly into gold (May-June 1897), announced by Emmens (Stephen - N. Emmeus), if the validity of the observations is justified and Argentaurum does not turn out to be a similar alchemical warning of the same kind, which has happened more than once and also hiding behind a cloak of secrecy and monetary interest. That cold and pressure can contribute to a change in structure and properties has long been known, at least from the example of Fritzsche’s tin, but there are no facts to suggest that these changes go so deeply and reach not to the structure of particles, but to what is now considered atoms and elements, and therefore the transformation (even if gradually) of silver into gold, affirmed by Emmens, will remain doubtful and insignificant even in relation to silver and gold, until, firstly, the “secret” is so revealed that the experience can be reproduced by everyone , and secondly, until the reverse transition (with heating and decreasing pressure?) of gold into silver is established, or until its actual impossibility or difficulty is established. It is easy to understand that the transition of alcohol carbon dioxide into sugar is difficult, although the reverse is easy, because sugar is undoubtedly more complex than alcohol and carbon dioxide. And it seems to me very unlikely that the transition of silver into gold, if vice versa, gold will not transform into silver, because the atomic weight and density of gold is almost twice that of silver, from which it should be concluded, based on everything known in chemistry, that if silver and gold came from the same material, then gold is more complex than silver and should be converted into silver more easily than back. Therefore, I think that Mr. Emmens, to be convincing, should not only reveal the “secret”, but also try and show, if possible, the transformation of gold into silver, especially since when receiving another from an expensive metal, 30 times more cheap, monetary interests will obviously be in the background, and the interests of truth and truth will clearly be in the first place, but now the matter seems, in my opinion, from the other side.
With this idea of chemical elements, they turn out to be something abstract, since we do not see or know them individually. Such a realistic knowledge as chemistry has arrived at such an almost idealistic idea from the totality of everything observed up to now, and if this idea can be defended, then only as an object of deeply rooted conviction, which until now has proven to be completely in agreement with experience and observation. In this sense, the concept of chemical elements has a deeply real basis in the entire science of nature, since, for example, carbon has never, never, been transformed into any other element, while a simple body - coal - has been transformed into graphite and diamond and, perhaps, someday it will be possible to turn it into a liquid or gaseous substance, if it is possible to find conditions for simplifying the most complex particles of coal. The main concept with which it is possible to begin to explain P.'s legality consists precisely in the fundamental difference in ideas about elements and about simple bodies. Carbon is an element, something unchangeable, contained both in coal and in carbon dioxide or in luminous gas, as in diamond, and in the mass of changeable organic substances, both in limestone and in wood. This is not a specific body, but a weighty (material) substance with a sum of properties. Just as in water vapor or in snow there is no specific body - liquid water, but there is the same weighty substance with the sum of properties belonging to it alone, so all carbonaceous matter contains materially homogeneous carbon: not coal, but precisely carbon. Simple bodies are substances containing only one element, and the concept of them becomes transparently clear only when the strengthened idea of atoms and particles or molecules from which homogeneous substances are composed is recognized; Moreover, the concept of an element corresponds to an atom, and to a simple body - a particle. Simple bodies, like all bodies of nature, are composed of particles: their only difference from complex bodies is that the particles of complex bodies contain heterogeneous atoms of two or many elements, and the particles of simple bodies contain homogeneous atoms of a given element. Everything that is stated below must relate specifically to the elements, i.e. eg to carbon, hydrogen and oxygen, as components of sugar, wood, water, coal, oxygen gas, ozone, etc., but not simple bodies formed by elements. At the same time, the question obviously arises: how can one find any real legitimacy in relation to such objects as elements that exist only as ideas of modern chemists, and what can actually be expected as a consequence of the investigation of some abstractions? Reality answers such questions with complete clarity: abstractions, if they are truthful (contain elements of truth) and correspond to reality, can serve as the subject of exactly the same study as purely material concreteness. Thus, chemical elements, although the essence of abstraction, are subject to investigation in exactly the same way as simple or complex bodies that can be heated, weighed and generally subjected to direct observation. The essence of the matter here is that chemical elements, on the basis of an experimental study of the simple and complex bodies they form, discover their individual properties and characteristics, the totality of which constitutes the subject of research. We will now turn to listing some of the features belonging to chemical elements in order to then show P. the legitimacy of chemical elements.
The properties of chemical elements should be divided into qualitative and quantitative, at least the first of them were themselves subject to measurement. Among the qualitative ones, first of all, is the ability to form acids and bases. Chlorine can serve as an example of the former, since with both hydrogen and oxygen it forms obvious acids that can form salts with metals and bases, starting with the prototype of salts - table salt. Sodium table salt NaCl can serve as an example of elements that produce only bases, since it does not produce acidic oxides with oxygen, forming either a base (sodium oxide) or a peroxide, which has the characteristic features of typical hydrogen peroxide. All elements are more or less acidic or basic, with obvious transitions from the former to the latter. Electrochemists (with Berzelius at their head) expressed this qualitative property of elements by distinguishing those similar to sodium, on the basis that the former, during decomposition, generate current at the anode, and the latter at the cathode. The same qualitative difference between elements is expressed partly in the distinction between metals and metalloids, since the basic elements are among those that form real metals in the form of simple bodies, and acidic elements form metalloids in the form of simple bodies that do not have the appearance and mechanical properties of real metals. But in all these relations, not only is direct measurement impossible, allowing one to establish the sequence of transition from one property to another, but there are also no sharp differences, so that there are elements in one relation or another that are transitional or those that can be attributed to both discharge. So aluminum, in appearance, is clearly a metal that is an excellent conductor of galvanic energy. current, in its only oxide Al 2 O 3 (alumina) plays either a basic or an acidic role, since it combines with bases (for example, Na 2 O, MgO, etc.) and with acidic oxides, for example, forming sulfur-alumina salt A1 2 (SO 4) 3 =Al 2 O 3 3O 3; in both cases it has weakly expressed properties. Sulfur, forming an undoubted metalloid, is similar in many chemical respects to tellurium, which, due to the external qualities of a simple body, has always been classified as a metal. Such cases, very numerous, give all the qualitative characteristics of the elements a certain degree of instability, although they serve to facilitate and, so to speak, revitalize the entire system of acquaintance with the elements, indicating in them signs of individuality that make it possible to predict the not yet observed properties of simple and complex bodies formed from elements. These complex individual characteristics of the elements gave extreme interest to the discovery of new elements, not allowing in any way to foresee the sum of physical and chemical characteristics characteristic of the substances formed by them. Everything that could be achieved in the study of elements was limited to bringing together the most similar ones into one group, which made all this acquaintance similar to the taxonomy of plants or animals, i.e. the study was slavish, descriptive and did not allow any predictions to be made in relation to elements not yet in the hands of researchers. A number of other properties, which we will call quantitative, appeared in their proper form for chemical elements only from the time of Laurent and Gerard, i.e. since the 50s of the current century, when the ability of mutual reaction on the part of the composition of particles was studied and generalized and the idea of two-volume particles was strengthened, i.e. that in the vapor state, while there is no decomposition, all particles (i.e., quantities of substances that enter into chemical interaction with each other) of all bodies occupy the same volume as two volumes of hydrogen occupy at the same temperature and the same pressure. Without going here into the presentation and development of the principles that were strengthened in this now generally accepted idea, it is enough to say that with the development of unitary or partial chemistry in the last 40 or 50 years, a hardness has emerged that previously did not exist, both in determining the atomic weights of elements and in determining the composition of the particles of simple and complex bodies formed by them, and the reason for the difference in the properties and reactions of ordinary oxygen O 2 and ozone O 3 became obvious, although both contain only oxygen, as well as the difference between oil gas (ethylene) C 2 H 4 from liquid cetene C 16 H 32, although both contain 12 parts by weight of carbon and 2 parts by weight of hydrogen. During this significant era of chemistry, two more or less precise quantitative characteristics or properties appeared in it for each well-examined element: the weight of the atom and the type (shape) of the particle composition of the compounds formed by it, although nothing had yet indicated either the mutual connection of these characteristics or on their relationship with other, especially qualitative, properties of elements. The atomic weight characteristic of an element, i.e. indivisible, the smallest relative amount of it, which is part of the particles of all its compounds, was especially important for the study of elements and constituted their individual characteristics, so far a purely empirical property, since to determine the atomic weight of an element it is necessary to know not only the equivalent or relative weight composition of some of it compounds with elements whose atomic weight is known from other definitions, or is conventionally accepted as known, but also determined (by reactions, vapor densities, etc. ) partial weight and composition of at least one, or better yet, many of the compounds it forms. This path of experiment is so complex, long and requires such completely purified and carefully studied material from among the compounds of the element that for many, especially for elements rare in nature, in the absence of particularly compelling reasons, there remained many doubts regarding the true value of the atomic weight, although the weight composition (equivalent) of some of their connections was installed; such, for example, were uranium, vanadium, thorium, beryllium, cerium, etc. Given the purely empirical value of the weight of an atom, there was no particular interest in delving into this subject for elements that are rarely subjected to research, nevertheless, for a large mass of ordinary elements of atomic size scales could already be considered firmly established at the beginning of the 60s, especially after Cannizzaro firmly established for many metals, for example. Ca, Ba, Zn, Fe, Cu, etc. their obvious difference from K, Na, Ag, etc., showing that particles e.g. chloride compounds of the former contain twice as much chlorine as the latter, i.e. that Ca, Ba, Zn, etc. give CaCI 2, BaCI 2, etc., i.e. diatomic (two-equivalent or divalent), while K, Na, etc. monoatomic (mono-equivalent), i.e. form KCI, NaCI, etc. Around the middle of this century, the weight of an atom of elements already served as one of the signs by which similar elements of groups began to be compared.
Another of the most important quantitative characteristics of elements is the composition of the particles of higher compounds formed by them. There is more simplicity and clarity here, because Dalton’s law of multiple ratios (or the simplicity and integrity of the number of atoms that make up the particles) already forces us to wait for only a few numbers and it was easier to understand them. The generalization was expressed in the doctrine of the atomicity of elements or their valence. Hydrogen is a monatomic element, because it gives one compound HX with other monatomic elements, of which chlorine was considered a representative, forming HCl. Oxygen is diatomic because it gives H 2 O or combines with two Xs, if by X we mean monatomic elements. This is how HClO, Cl 2 O, etc. are obtained. In this sense, nitrogen is considered triatomic, since it gives NH 3, NCl 3; carbon is tetraatomic because it forms CH 4, CO 2, etc. Similar elements of the same group, e.g. halogens also give similar particles of compounds, i.e. have the same atomicity. Through all this, the study of the elements has advanced greatly. But there were many difficulties of various kinds. Oxygen compounds, as a diatomic element capable of replacing and retaining X2, presented a particular difficulty, making the formation of Cl2O, HClO, etc. completely understandable. compounds with monatomic elements. However, the same oxygen produces not only HClO, but also HClO 2, HClO 3 and HClO 4 (perchloric acid), just like not only H 2 O, but also H 2 O 2 (hydrogen peroxide). To explain, we had to admit that oxygen, due to its diatomicity, having two affinities (as they say), is capable of squeezing into every particle and standing between any two atoms included in it. There were a lot of difficulties, but let’s focus on two, in my opinion, the most important. Firstly, it turned out that there was a kind of O 4 edge for the number of oxygen atoms included in the particle, and this edge cannot be expected based on what was assumed. Moreover, approaching the edge, the resulting connections were often not less, but stronger, which is no longer possible at all when thinking about squeezed oxygen atoms, since the more of them there are, the more likely it was to have weak bonds. Meanwhile, HClO 4 is stronger than HClO 3, this latter is stronger than HClO 2 and HClO, while HCl is again a chemically very strong body. The O 4 facet appears in the fact that hydrogen compounds of different atomicities:
HCl, H 2 S, H 3 P and H 4 Si
Higher oxygen acids answer:
HClO 4, H 2 SO 4, H 3 PO 4 and H 4 SiO 4,
which equally contain four oxygen atoms. From this even comes the unexpected conclusion that considering H as mono- and O as diatomic elements, the ability to combine with oxygen is the opposite of that with hydrogen, i.e. as elements increase in their ability to hold hydrogen atoms or increase in atomicity, their ability to hold oxygen decreases; chlorine, so to speak, is monoatomic in hydrogen and semiatomic in oxygen, and phosphorus or its analogous nitrogen is triatomic in the first sense, and pentaatomic in the second, as can be seen in other compounds, for example NH 4 CI, POCl 3, PCl 5, etc. .P. Secondly, everything that we know clearly points to a profound difference in the addition of oxygen (squeezing it in, judging by the idea of the atomicity of elements) in the case when hydrogen peroxide is formed, from when, for example, it occurs. from H 2 SO 4 (sulphurous acid) sulfuric acid H 2 SO 4, although H 2 O 2 differs from H 2 O in exactly the same oxygen atom as H 2 SO 4 from H 2 SO 3, and although deoxidizers in both cases convert the highest oxidation state to the lowest. The difference in relation to the reactions characteristic of H 2 O 2 and H 2 SO 4 is especially pronounced due to the fact that sulfuric acid has its own peroxide (persulfuric acid, the analogue of which, perchromic acid, was recently studied by Wiede and contains, according to his data, H 2 CrO 5 ), which has all the properties of hydrogen peroxide. This means that there is a significant difference in the method of addition of oxygen in “salt-like” oxides and real peroxides and, therefore, simply squeezing oxygen atoms between others is not enough to express all cases of oxygen addition, and if expressed, then most likely it should be applied to peroxides, and not to the formation, so to speak, of normal oxygen compounds approaching RH n O 4, where n, the number of hydrogen atoms, does not exceed 4, just like the number of oxygen atoms in acids containing one atom of the elements R. Taking what has been said into account and generally meaning through R atom of elements, the entire body of information about salt-like oxides leads to the conclusion that the number of independent forms or types of oxides is very small and is limited to the following eight:
R 2 O 2 or RO, e.g. CaO, FeO.
This harmony and simplicity of oxidation forms does not at all follow from the doctrine of the atomicity of elements in its usual form (when determining atomicity by a compound with H or Cl) and is a matter of direct comparison of oxygen compounds themselves. In general, the doctrine of the constant and unchanging atomicity of elements contains difficulties and imperfections (unsaturated compounds like CO, supersaturated ones like JCl 3, compounds with water of crystallization, etc.), but in two respects it is still important today , namely, with it, simplicity and harmony in the expression of the composition and structure of complex organic compounds has been achieved, and in relation to the expression of the analogy of related elements, since atomicity, no matter how it is considered (or the composition of particles of similar compounds), in this case turns out to be the same. So eg. halides or metals of a given group that are similar to each other in many other ways (alkali, for example) always turn out to have the same atomicity and form a whole series of similar compounds, so the existence of this feature is already, to some extent, an indicator of analogy.
In order not to complicate the presentation, we will leave the enumeration of other qualitative and quantitative properties of elements (for example, isomorphism, heat of connection, display, refraction, etc.) and directly turn to the presentation of the P. law, for which we will dwell: 1) on the essence of the law , 2) on its history and application to the study of chemistry, 3) on its justification with the help of newly discovered elements, 4) on its application to the determination of the value of atomic weights and 5) on some incompleteness of existing information.
The essence of P. legality. Since of all the properties of chemical elements, their atomic weight is the most accessible for numerical accuracy of determination and for complete convincingness, then the most natural outcome for finding the validity of chemical elements is to put the weights of atoms, especially since in weight (according to the law of conservation of mass) we are dealing with indestructible and the most important property of all matter. The law is always a correspondence of variables, just as in algebra their functional dependence. Consequently, having atomic weight for elements as one variable, to find the law of elements one should take other properties of elements as another variable and look for functional dependence. Taking many properties of elements, e.g. their acidity and basicity, their ability to combine with hydrogen or oxygen, their atomicity or composition of their respective compounds, the heat released in the formation of the corresponding, e.g. chloride compounds, even their physical properties in the form of simple or complex bodies of similar composition, etc., one can notice a periodic sequence depending on the atomic weight. In order to find out, let us first present a simple list of all the currently well-known definitions of the atomic weight of elements, guided by a recent compilation made by F.W. Clarke (Smithsonian Miscellaneous Collections, 1075: “A recalculation of the atomic weights”, Washington, 1897, p. 34), since it should now be considered the most reliable and contains all the best and latest definitions. In this case, we will accept, together with the majority of chemists, the conditional atomic weight of oxygen equal to 16. A detailed study of the “probable” errors shows that for approximately half of the given results the error in numbers is less than 0.1%, but for the rest it reaches several tenths, and for others , perhaps up to a percentage. All atomic weights are given in order of magnitude.
Conclusion
The periodic system of Dmitry Ivanovich Mendeleev was of enormous importance for natural science and all science in general. She proved that man is capable of penetrating the secrets of the molecular structure of matter, and subsequently - the structure of atoms. Thanks to the successes of theoretical chemistry, a whole revolution was accomplished in industry, and a huge number of new materials were created. The relationship between inorganic and organic chemistry was finally found - the same chemical elements were discovered in both the first and second.
Here one colleague thought that Dmitry Ivanovich Mendeleev was “one of the rabbis.” Like, he has a rabbinical beard.
It’s a strange association, although, yes, the beard looks like Karlo-Marx’s, and he really was the grandson of as many as two rabbis.
And personally, since school, I have been puzzled by the obvious discrepancy between Mendeleev’s affairs, his name, appearance on the one hand and... his purely Jewish surname on the other! Look at the portrait below: what is Semitic or Jewish there? A Russian man with... a falcon's gaze!
Thanks to my colleague evstoliya_3 , (who once unfriended me, most likely for criticizing the Russian Orthodox Church), which is a link to interesting material about Dmitry Ivanovich. Where, by the way, the falcon gaze of the Russian scientist is clearly explained.
And near Yaroslavl, in the village of Konstantinovo, there is a small oil refinery (built by my great-great-grandfather Viktor Ivanovich Ragozin). There is still an interesting factory museum there, where a lot of materials are devoted to the period of Mendeleev’s work in the laboratory of the enterprise. There is absolutely original materials.
The museum was created by many years of efforts of a remarkable devotee in preserving Russian history. Galina Vladimirovna Kolesnichenko. Who gave him, in fact, her entire working life. Galina Vladimirovna is also the author of an interesting monograph about the Russian oleonaft Viktor Ivanovich and about the Ragozin family in general. Almost 800 pages, excellent design, circulation only... one hundred copies ( Ragozin brothers. The beginning of the Russian oil business: A documentary biographical story.- St. Petersburg: Alpharet, 2009. - 756 p.).
And now - "".
*
It is unusual for a Russian person to waste his time on trifles.
What is the matter here - whether there are huge spaces, whether there is winter for six months, or the absence of roads, but it was in our fatherland that citizens preferred to immediately attack the foundations of the universe.
It would seem that it would be better for the Kaluga teacher to improve the hearing aid, which he desperately needed, but no, Tsiolkovsky took up interplanetary travel and the settlement of other planets.
The excellent geochemist Vernadsky - not to continue studying pebbles - came up with some kind of intelligent layer on planet Earth, the noosphere. Chizhevsky explained literally all events on Earth by the influence of the Sun.
In short, I don’t want to dig into the little things in Russia; let the Germans do that.
And in our country it is customary to create comprehensive - and most often ridiculous - theories with a minimum of experimental data.
But miracles sometimes happen, if only a suitable genius could be found. This is how Dmitry Ivanovich Mendeleev was.
Everyone knows that he discovered the periodic table of chemical elements.
Many people remember that he theoretically and practically substantiated the optimal strength of vodka. But only about 9% of his more than 500 scientific works are devoted to chemistry.
And how many other hobbies did this brilliant man have besides science!
Dmitry Ivanovich Mendeleev was born on January 27 (February 8), 1834 in the village of Verkhnie Aremzyany not far from Tobolsk, the seventeenth and last child in the family of Ivan Pavlovich Mendeleev, who at that time held the position of director of the Tobolsk gymnasium and schools of the Tobolsk district.
Dmitry's paternal grandfather was a priest and bore the surname Sokolov; Dmitry's father received the surname Mendeleev in theological school in the form of a nickname, which corresponded to the customs of that time.
Mendeleev's mother came from an old but impoverished merchant family, the Kornilievs.
Having graduated from the gymnasium in Tobolsk in 1849, due to territoriality, Mendeleev could only enter Kazan University in Russia. But he never became a student of N.N. Zinin. Since Moscow and St. Petersburg universities were closed to him, he entered the St. Petersburg Pedagogical Institute in the department of natural sciences of the Faculty of Physics and Mathematics.
And I was right. Outstanding scientists of that time taught there - M.V. Ostrogradsky (mathematics), E.Kh. Lenz (physics), A.N. Savich (astronomy), A.A. Voskresensky (chemistry), M.S. Kutorga (mineralogy), F.I. Ruprecht (botany), F.F. Brandt (zoology).
While still a student in 1854, Dmitry Ivanovich conducted research and wrote an article “On isomorphism,” where he established the relationship between the crystalline form and chemical composition of compounds, as well as the dependence of the properties of elements on the size of their atomic volumes. In 1856 he defended his dissertation “On specific volumes” for a master’s degree in chemistry and physics.
At this time he writes about enanthic sulphurous acid and the difference between substitution, combination and decomposition reactions.
In 1859, Mendeleev was sent abroad. In Heidelberg he studied the capillarity of liquids. He discovered the “absolute boiling point of liquids,” or critical temperature, in 1860.
Returning, in 1861 he published the first Russian textbook “Organic Chemistry”. In 1865-1887 he created the hydration theory of solutions. Developed ideas about the existence of compounds of variable composition. In 1865 he bought the Boblovo estate, where he conducted research on agrochemistry and agriculture.
In 1868, together with Zinin and other scientists became the founder of the Russian Physical and Chemical Society.
In 1869, Dmitry Ivanovich Mendeleev made the greatest discovery in the history of chemistry - he created the famous periodic table of elements. In 1871, his book “Fundamentals of Chemistry” was published - the first harmonious presentation of inorganic chemistry. Mendeleev worked on new editions of this work until the end of his life.
About creating a table:
He bought about seventy blank business cards and on each of them he wrote on one side the name of the element, and on the other - its atomic weight and the formulas of its most important compounds. After that, he sat down at a large square table and began to lay out these cards in every way. At first, nothing worked for him.
Dozens and hundreds of times he laid them out, shuffled them and laid them out again. At the same time, as he later recalled, some new patterns emerged in his mind, and with the well-known excitement that precedes a discovery, he continued his work.
So he spent whole hours and days, locked in his office. Fortunately, by that time he was already married to Anna Grigorievna, who managed to create for him the best conditions for creative pursuits.
The legend that the idea of the periodic table came to him in a dream was invented by Mendeleev specifically for persistent fans who do not know what creative insight is. In fact, it just dawned on him. In other words, it immediately and finally became clear to him in what order the cards should be laid out so that each element would take its rightful place, according to the laws of nature.
In 1871-1875, Mendeleev studied the properties of elasticity and expansion of gases, explored petroleum hydrocarbons and questions of the origin of oil, about which he wrote several works. Visits the Caucasus. In 1876 he went to America, to Pennsylvania, to inspect American oil fields. Mendeleev's work in terms of studying oil production was of great importance for the rapidly developing oil industry in Russia.
The result of one of the then fashionable hobbies was the study “On Spiritualism.”
Since 1880, he began to be interested in art, especially Russian, collecting art collections, and in 1894 he was elected a full member of the Imperial Academy of Arts. His portrait is painted by Repin.
Since 1891, Mendeleev became the editor of the chemical-technical and factory department of the Brockhaus and Efron Encyclopedic Dictionary and wrote many of the articles himself. As a hobby, Dmitry Ivanovich made suitcases and sewed his own clothes. Mendeleev also participated in the design of the first Russian icebreaker Ermak.
In 1887, Mendeleev independently ascended in a balloon to observe a solar eclipse. The flight was unprecedented and became famous throughout the world. This is how G. Chernechenko describes this case in issue 8 of one of the newspapers dated August 19, 1999 (the article is called: “Mendeleev in a Balloon”):
In the small picturesque estate of D.I. Mendeleev Boblovo prepared to observe a solar eclipse at home. And suddenly, when a little more than a week remained before the eclipse, a telegram arrived from St. Petersburg to Boblovo. In it, the Russian Technical Society announced that a balloon was being equipped in Tver to observe the eclipse and that the council considered it its duty to declare this so that Mendeleev, if desired, “could personally take advantage of the rise of the balloon for scientific observations.”
Actually, neither the flight itself nor the invitation to participate in it was a big surprise for Mendeleev. Only one thing confused the great chemist: a ball filled with illuminating gas (there was no other gas in Tver) could not rise above two miles, and, therefore, would remain captive of the clouds. What was needed was a balloon filled with light hydrogen. He reported this in an urgent telegram that left Boblovo for the capital.
It was getting light. It was cloudy and drizzling. In the vacant lot between the railway line and the station, a ball was swaying, surrounded by a fence of poles. Nearby stood a gas production plant manned by soldiers in acid-stained shirts.
“We were waiting for Professor Mendeleev. At 6:25 a.m. there was applause, and a tall, slightly stooped man with graying hair hanging over his shoulders and a long beard came out of the crowd to the ball. It was the professor,” Vladimir told readers of Russkie Vedomosti Gilyarovsky.
The minute of the eclipse was approaching. Last goodbyes. Tall, slender Kovanko is already in the basket. Mendeleev in a brown coat and hunting boots makes his way there with difficulty through a web of ropes.
“For the first time I entered the basket of the ball, although, however, I once ascended in Paris in a tethered balloon. Now we were both in place,” the scientist later said
Further events unfolded in a matter of seconds. Everyone suddenly saw how Mendeleev said something to his companion, how Kovanko jumped out of the basket, and the ball slowly went up. A stool and a board that served as a table flew overboard. As luck would have it, the damp ballast turned into a dense lump. Having sunk to the bottom of the basket, Mendeleev threw wet sand down with both hands.
The unexpected flight of Mendeleev alone, the disappearance of the ball in the clouds and the sudden darkness, according to Gilyarovsky, “had a depressing effect on everyone, it became somehow eerie.” Anna Ivanovna was taken home to the estate, numb with horror. The painful atmosphere intensified when someone sent an incomprehensible telegram to Klin: “The ball was seen - Mendeleev is not there.”
Meanwhile, the flight was successful. The ball rose to a height of more than three kilometers, broke through the clouds, and Mendeleev managed to observe the total phase of the eclipse. True, before the descent the scientist had to show not only fearlessness, but also dexterity. The rope coming from the gas valve is tangled. Mendeleev climbed onto the side of the basket and, hanging over the abyss, unraveled the valve rope.
The balloon landed safely in the Kalyazinsky district of the Tver province, the peasants escorted Mendeleev to a neighboring estate.
The news of the unusually daring flight of the Russian professor soon became known to the whole world.
The French Academy of Meteorological Aeronautics awarded Mendeleev a diploma “For his courage during the flight to observe a solar eclipse.”
In 1888, on instructions from the government, he studied the causes of the crisis in the coal industry in the Donetsk region. His works “Letters on Factories” and “Intelligible Tariff” contained important economic proposals.
In 1890-1895 he was a consultant to the Scientific and Technical Laboratory of the Naval Ministry. In 1892 he organized the production of the smokeless gunpowder he invented.
In 1892, Mendeleev was appointed scientist-custodian of the Depot of Model Weights and Scales. Since 1893, on his initiative, it has become the Main Chamber of Weights and Measures. Now it is the All-Russian Research Institute of Metrology named after. DI. Mendeleev. As a result, already in 1899 a new law on weights and measures was introduced in Russia, which contributed to the development of industry.
For one of his anniversaries, Dmitry Ivanovich was given precious chemical scales made of pure aluminum - the electrochemical method for producing this cheap metal was unknown at that time, although Mendeleev’s works also indicate this technology.
American physicists synthesized the 101st element of the table and called it mendelevium; on Earth there is a mineral named after Mendeleev, a volcano and an underwater mountain range of Mendeleev, and on the far side of the Moon there is the Mendeleev crater.
Jokes are told only about the greats
There has been a whole series of anecdotes about Dmitry Ivanovich Mendeleev. Some stories really happened, while others were clearly made up.
For example, there is a story about a visit to Mendeleev’s laboratory by one of the great princes. The famous chemist, in order to point out the plight of the laboratory and get money for research, ordered to fill up the corridor along which the prince was supposed to walk with all sorts of junk and boards from the fence. The prince, inspired, released some funds.
Another story that has become a classic is related to Mendeleev’s hobby - making suitcases. One day, a driver with a rider in a carriage suddenly rose from his seat, bowed and raised his hat to some passer-by. The surprised rider asked: “Who is this?” “Oh!” replied the cabman. This is the famous suitcase master Mendeleev!“It should be noted that all this happened when Dmitry Ivanovich was already an internationally recognized great scientist.
And once, in almost similar circumstances, the cab driver respectfully informed the rider that he was the chemist Mendeleev. "Why isn't he arrested?" - the rider was surprised. The fact is that in those years the word “chemist” was synonymous with the word “swindler”.
The legend of the invention of vodka
In 1865, Dmitry Mendeleev defended his doctoral dissertation on the topic “Discourse on the combination of alcohol with water,” which had nothing to do with vodka. Mendeleev, contrary to the prevailing legend, did not invent vodka; it existed long before him.
The label of the “Russian Standard” states that this vodka “meets the standard of Russian vodka of the highest quality, approved by the Tsarist government commission headed by D. I. Mendeleev in 1894.” The name of Mendeleev is associated with the choice of vodka with a strength of 40°. According to the Vodka Museum in St. Petersburg, Mendeleev considered the ideal strength of vodka to be 38°, but this number was rounded to 40 to simplify the calculation of alcohol taxes.
However, it is not possible to find a justification for this choice in the works of Mendeleev. Mendeleev's dissertation on the properties of mixtures of alcohol and water does not distinguish 40° or 38°. The “Tsarist Government Commission” could not establish this standard for vodka, if only because this organization - the Commission for finding ways to streamline the production and trade circulation of drinks containing alcohol - was formed at the suggestion of S. Yu. Witte only in 1895 Moreover, Mendeleev spoke at its meetings at the very end of the year and only on the issue of excise taxes.
Where did 1894 come from? Apparently, from an article by historian William Pokhlebkin, who wrote that “30 years after writing the dissertation... agrees to join the commission.” The manufacturers of the “Russian Standard” added a metaphorical 30 to 1864 and obtained the desired value.
Vodka with a strength of 40° became widespread already in the 16th century. It was called polugar because when burned its volume was halved. Thus, checking the quality of vodka was simple and publicly available, which became the reason for its popularity.
“I myself am surprised,” Mendeleev wrote at the end of his life, “what I have not done in my life. And I think it was done well.” He was a member of almost all academies and an honorary member of more than 100 learned societies.
Mendeleev conducted and published fundamental research in chemistry, chemical technology, pedagogy, physics, mineralogy, metrology, aeronautics, meteorology, agriculture, and economics. All his works were closely related to the needs of the development of productive forces in Russia.
At the beginning of the 20th century, Mendeleev, noting that the population of the Russian Empire had doubled over the past forty years, calculated that by 2050 its population would reach 800 million people.
In January 1907, D.I. Mendeleev himself caught a bad cold while showing the House of Weights and Measures to the new Minister of Industry and Trade Filosofov.
First, dry pleurisy was diagnosed, then doctor Yanovsky found Dmitry Ivanovich to have pneumonia. On January 19, at 5 o'clock, the great Russian chemist passed away. He was buried next to his son at the Volkovskoye cemetery in St. Petersburg. He bought this place for himself shortly after the death of his son; it was located near the grave of D.I. Mendeleev’s mother.
“Mendeleev... accomplished a scientific feat that can safely be placed next to the discovery of Le Verrier, who calculated the orbit of a still unknown planet - Neptune.”
F. ENGELS
Was there order or was there no order?
In the second half of the last century, science has already received quite a lot of information about the behavior of thyroid atoms. The patterns of transformations of elements became clear. Even the great Russian scientist M.V. Lomonosov argued that nature is not a chaotic accumulation of processes: certain patterns appear in it. Understanding and using these patterns is the task of science.
This statement of Lomonosov became more and more confirmed with each passing decade. It especially well confirmed Dalton's theory, developed by Avogadro and Berzelius. Thanks to the work of these scientists, no one doubted that the entire variety of transformations and properties of substances depends on the behavior of the smallest particles - atoms.
Dozens of chemical elements were already known and it was precisely established that from these elements, the atoms of which are combined in chemical reactions in a certain way, all other substances are obtained.
But nevertheless, it remained unclear: why do some elements behave this way, others differently? Why do some elements exhibit approximately the same properties, but their atomic weights are very different? Why are some heavier and others lighter? And there were many such “whys”.
There was no real order in the world of substances yet. Or rather, there was order, Lomonosov had already predicted, but what it was, what the laws of this order were, was unclear.
March sensation
It happened on March 6, 1869. On that day, a meeting of the Russian Physicochemical Society took place at St. Petersburg University. The most prominent Russian scientists present at the meeting already knew approximately the topic of the message that would be made at the meeting. The author of this message was a young talented professor at the Department of Inorganic Chemistry of St. Petersburg University, Dmitry Ivanovich Mendeleev.
As early as January 1869, many of the scientists present at this meeting received a sheet entitled “An experiment on a system of elements based on their atomic and chemical similarities.”
The symbols of chemical elements were written on the sheet. There were 63 of them known at that time. Scientists noticed that the chemical elements in this small tablet are arranged in order of increasing atomic weights. But not everyone then understood that this was the great meaning of Mendeleev’s short note.
But what they heard at the meeting was a huge sensation. True, Mendeleev himself was not at the meeting. He was sick that day. Professor N.A. Menshutkin made a message on his behalf. The title of the message was “Relationship of properties with atomic weight of elements.” What was described in the message was a great discovery that had a huge impact on science. After Mendeleev's discovery, a new era in the development of science began - the era of atomic science. And that's why.
Is it possible to make a great discovery by accident?
When Mendeleev reported the relationship between the properties of elements and their atomic weights, he was 35 years old. He was already a fairly well-known chemist at that time; he was well versed in the intricacies of chemical transformations of elements and the peculiarities of reactions. In 1867
Dmitri Ivanovich Mendeleev.
Mendeleev began writing the book “Fundamentals of Chemistry”. And the further the work progressed, the more he thought about presenting the material of the book, the more he felt some kind of dissatisfaction.
He saw that numerous chemical reactions, properties of elements and much more are not united by a single meaning, a single “core”. Something was missing.
Gradually, more and more often he began to think: is there a pattern between the atomic weights of elements and their properties? In order to more clearly identify this pattern, Mendeleev wrote on separate cards the names of the elements, their atomic weight and basic chemical properties. After that, he began to lay out the cards in a certain order according to increasing atomic weights of the elements.
Hydrogen came first. Its atomic weight is equal to one. Other elements followed. The result was a chain of 63 cards (according to the number of elements known at that time). So what? There is no pattern. What if you select columns of elements that form identical compounds with oxygen and distribute them so that the elements are arranged in the order of atomic weights on the lines of cards? Mendeleev did this, and it became clear to him that elements with the same chemical properties are grouped in a certain sequence.
I had to analyze, group, and study the arrangement of elements many more times, but now it was clear: the chemical properties of elements arranged as their atomic weights increase are repeated! This is how the periodic law of the elements was discovered.
And, of course, this is not an accidental discovery. Only enormous knowledge, experience and a well-developed sense of scientific foresight allowed Mendeleev to establish that atomic weight is the main characteristic reflecting the diversity of properties of elements.
First results
Of the 63 cards that Mendeleev laid out, nine did not correspond to the pattern of the table. What's the matter? So the law is wrong? No, Mendeleev firmly believed in the power of the law and did not doubt its correctness. Since the cards fall out of the general pattern, it means that the atomic weights of these elements were determined incorrectly. This means that these elements need to be placed where elements similar to them in chemical properties are located. Knowing the atomic weights of neighboring ones, one can obtain the atomic weight of these elements that “do not obey” the law. This is how the atomic weights of beryllium, indium, thorium, and uranium were corrected. True, Mendeleev did not do this immediately, but some time after his message, when he continued to improve the table. More accurate experiments carried out later allowed scientists to verify that, indeed, the initially determined atomic weights of the elements turned out to be incorrect. Their atomic weights exactly matched those predicted by Mendeleev.
But that's not all. When Mendeleev compiled the table, some places in it were left blank. Convinced of the correctness of the periodic law he discovered, Mendeleev boldly assumed that there must be elements here that had not yet been discovered. He named them ekaboron, ekasilicon, and ekaaluminium (the prefix “eka” meant that the element was similar to boron, silicium, or aluminum) and argued that such elements must exist.

And indeed, in August 1875, a new element was discovered - gallium. When its properties were determined, it turned out that this was eka-aluminium, predicted by Mendeleev. Four years later, another element was found, predicted by Mendeleev and called eca-boron. It was called scandium. Seven years later, a third element was found - eca-silicon. He received the name germanium.
Thus the correctness of the law discovered by Mendeleev was brilliantly confirmed
Mendeleev's thoughts on the structure of the atom
Mendeleev was a chemist. But for a chemist, the main thing is the chemical individuality of the elements. Mendeleev's great merit lies in the fact that he was the first to establish the carriers of this individuality - atoms. He emphasized that atoms are indivisible in the chemical sense, “just as when people consider the relations between them, a person is an indivisible unit.”
But this individuality of atoms, as Mendeleev taught, is explained by their deep and complex structure of “internal movements.” In other words, the scientist considered the concept of “motion” to be inextricably linked with the concept of “matter”; Mendeleev believed that “the world of atoms is structured in the same way as the world of heavenly bodies, with its suns, planets and satellites.”
Moreover, Mendeleev made a very bold assumption that when atoms are formed, energy should be released and their weight should change. The further development of science confirmed this precisely when scientists became aware of the first nuclear reactions.

Chapter 3. The structure of the atom Radioactivity A brilliant series of physical discoveries in the last decade of the 19th century truly marked the beginning of a scientific revolution. The prologue to it was the discovery made in 1896 by the French physicist Antoine Henri Becquerel, who
From the book Nuclear Energy for Military Purposes author Smith Henry DewolfRADIOACTIVITY AND ATOMIC STRUCTURE 1.6. The phenomena of radioactivity, discovered by A. Becquerel in 1896 and subsequently studied by Pierre and Marie Curie, E. Rutherford and many others, played a leading role in the discovery of the general laws of atomic structure and in confirming the equivalence
From the book Course in the History of Physics author Stepanovich Kudryavtsev PavelModels of the atom before Bohr The development of research into radioactive radiation, on the one hand, and quantum theory, on the other, led to the creation of the Rutherford-Bohr quantum model of the atom. But the creation of this model was preceded by attempts to build a model of the atom based on
From the book E=mc2 [Biography of the most famous equation in the world] by Bodanis DavidChapter 8 Inside the Atom University students in 1900 were taught that ordinary matter—the stuff that makes up bricks and steel and uranium and everything else—was itself made up of tiny particles called atoms. However, no one knew what atoms were made of. General opinion
From the book Where the River of Time Flows author Novikov Igor DmitrievichFIRST THOUGHTS ABOUT TIME For a long time, when I began to read popular books on physics, it seemed self-evident to me that time is empty duration, flowing like a river, carrying away all events without exception. It invariably and inevitably flows in one
From the book What the Light Tells About author Suvorov Sergei GeorgievichThe spectrograph confirms Mendeleev's predictions. During these same years, the great Russian scientist D.I. Mendeleev (1834-1907) studied the connection between the chemical properties of elements and their atomic weights. He found that if all the elements were arranged in one row according to the increasing weights of their atoms, starting with
From the book History of the Laser author Bertolotti MarioWhat is the structure of an atom Model of the hydrogen atom In 1913, the Danish physicist Niels Bohr (1885-1962) tried to paint a clear picture: how an atom can be built from a positive nucleus and electrons and under what conditions it emits light. Physicists call this visual
From the book The Atomic Problem by Ran PhilipModel of the hydrogen atom In 1913, the Danish physicist Niels Bohr (1885-1962) tried to paint a clear picture of how an atom could be built from a positive nucleus and electrons and under what conditions it emits light. Physicists call such a visual picture a model of an atom. Problem
From the book The King's New Mind [On computers, thinking and the laws of physics] by Penrose RogerThe exact place of elements in the periodic table Some chemical elements are not listed in the periodic table in order of increasing atomic weights. These are the three groups of elements: No. 18 - argon (atomic weight 39.9) and No. 19 - potassium (its atomic weight is less - 39.1), then No. 27 - cobalt (atomic weight
From the book Scientific Ideas by A.D. Sakharov today author Altshuler Boris LvovichThe first model of the atom In conclusion, we can say that in the first years of the 20th century. the first, perhaps incomplete, answer to the question of how light was emitted was given, and atoms with their electrical charges were considered responsible. However, how atoms are structured and, accordingly, what are
Some parting thoughts Every time I watch Interstellar or leaf through the manuscript of this book, I am amazed at the enormous variety and beauty of the scientific concepts contained in it. And what excites me most is the optimistic message inherent in
We present to your attention another article in our “Lives of Remarkable Minds” series.
At the next meeting of the Russian Chemical Society, held on March 6, 1869, Dmitry Ivanovich Mendeleev was not present. He was quite unexpectedly called to one of the recently opened chemical plants. Therefore, his report “Relationship of properties with the atomic weight of elements” was read by his friend, the first editor of the journal RHO Nikolai Aleksandrovich Menshutkin. The assembled scientists calmly listened to the speaker, politely clapped him and slowly dispersed. Everything was as if nothing had happened, and the world after this report remained the same as it was before it.
Now even schoolchildren know that Mendeleev saw his periodic table in a dream. And it cannot be said that this information is not true. At least, the scientist himself talked about how, after three days of painful reasoning, he fell asleep. And suddenly: “I clearly see in a dream a table where the elements are arranged as needed. I woke up, immediately wrote it down on a piece of paper and fell asleep again. Only in one place was an amendment subsequently necessary.” Later, when the significance of the discovery became clear to all educated people, journalists greedy for sensations spread the word about it all over the world. This, they say, is how great theories are obtained: a man lay down, fell asleep, saw something and woke up as a great discoverer. Finally, in response to another request to tell how it is possible to see such a useful thing as the “Periodic Table” in a dream, this time from a reporter for “Petersburg Leaflet”, the scientist could not stand it and exploded: “...Not a nickel for a line ( standard newspaper fee, - V.Ch.)! Not like you! I’ve been thinking about it for maybe twenty-five years, and you think: I was sitting there, and suddenly a nickel for a line, a nickel for a line, and it’s done...!”
This story about a sudden “dream epiphany” was just one of the few legends that popular, literary and newspaper rumors associated with the name of the great scientist. In total there was a great mass of them.
Although Dmitry Ivanovich was born into a cultural family with ancient traditions, his surname cannot be called ancient. His grandfather, the rural parish priest Pavel Maksimovich, was Sokolov. And only one of the four sons, Timothy, remained in his surname; the other three, according to the customs of the clergy of that time, were given different surnames after graduating from the seminary. The first, Alexander, after the name of the village where his father served, became Tikhomandritsky, the second, Vasily, after the name of the parish - Pokrovsky, and the third, Ivan, was given the name of the neighbors and permanent parishioners of the Sokolovs - the landowners Mendeleevs. After graduating from theological school, Ivan went along the secular line, studied at the philological department of the St. Petersburg Main Pedagogical Institute, which later became the State University, after which he was appointed “teacher of philosophy, fine arts and political economy” in Tobolsk. Already there he married the merchant’s daughter Maria Dmitrievna Kornilieva, who bore him 17 children. The seventeenth, “last one,” on January 27, 1834, was Dmitry. Although, if you count differently, he was the ninth, since eight died in infancy.
By that time, the Mendeleev family had reached the peak of its economic prosperity: Ivan Pavlovich was already the director of the Tobolsk gymnasium and schools of the Tobolsk district. But this prosperity collapsed instantly. In the same 1834, Dmitry’s father became blind due to cataracts and retired, the amount of which was extremely small.
Here, the entrepreneurial acumen of Mendeleev’s mother, inherited from her father, came in handy. She moved her family to the village of Aremzyanskoye, where her brother had a small glass factory. The brother lived permanently in Moscow, and completely entrusted the management of the enterprise to Maria. In 1841, Mitya was sent to the Tobolsk gymnasium. Another well-known legend is associated with this period, which is often consoled by losers. Everyone knows that Mitya Mendeleev, a future brilliant scientist, was kept in the gymnasium for the second year. This was really so, only they left him not because of poor academic performance, but because they sent him there not at the age of 8, as was expected, but at 7. Just on the condition that he would study in first grade two year in a row.
In 1847, Ivan Pavlovich died, and then all the worries of providing for a rather large family fell entirely on the shoulders of Maria Dmitrievna. She tried to give all her children the best possible education, and when the last one, Dima, graduated from high school, she completed all her “glass business,” sold everything she had in Tobolsk and moved to St. Petersburg with her son and youngest daughter. Where, at her persistent request, Dmitry was enrolled in the same Pedagogical Institute from which his father graduated, only to the Faculty of Physics and Mathematics. However, the young student gave greater preference, as one might already guess, to chemistry and mineralogy, taught by the famous professors “grandfather of Russian chemistry” Alexander Voskresensky and Stepan Kutorga. Under their guidance, in 1854 he published his first serious work, “Chemical Analysis of Orthite from Finland.”
A year later, Mendeleev graduated from the institute with a gold medal, received the title “Senior Teacher” and left cold St. Petersburg to teach in warm Odessa, where he worked for a year at the Richelieu Lyceum. However, here he did not so much teach as he worked on his master’s thesis on the topic “Structure of silica compounds,” which he defended in 1856. The dissertation was a success; as a result of the defense, Mendeleev received a master's degree and the position of private assistant professor at St. Petersburg University.
In 1859, “to improve his science,” the young promising chemist was sent to Heidelberg, Germany, where he studied the relationship between the chemical and physical properties of substances for two years. In this area, he was able, in particular, to prove that there is a maximum temperature at which any substance can only be in a gaseous state. Returning to St. Petersburg, he soon wrote and published a wonderful textbook on organic chemistry, which brought him considerable fame in enlightened circles.
In the spring of 1863, he married the stepdaughter of the famous writer, author of “The Little Humpbacked Horse” Pyotr Ershov, who, by the way, taught him literature at the gymnasium, Feozva Nikitichna Leshcheva. She was 6 years older than her husband and brought him three children. At the same time, he was awarded a very decent Demidov Prize for “Organic Chemistry,” and a little later he took up the position of full-time associate professor at the Department of Organic Chemistry at St. Petersburg University with a solid salary of 1,200 rubles a year. At the same time, he simultaneously received a position as a professor and, as a professor, an apartment at the institute. Thus, all the financial problems that tormented young families were largely removed and the scientist could devote himself to chemical research with a pure heart.
For more than a year he studied the alcohol-water mixture and eventually came to the conclusion that the solution with the highest density is one in which there is one C2H5OH for every three H2O molecules. In 1865, he defended his doctoral dissertation on the topic “Discourse on the combination of alcohol with water.” It flows organically another legend claiming that it was Mendeleev who invented Russian vodka. The legend even says that “in his dissertation, Dmitry Ivanovich convincingly proved that the optimal strength of “life-giving water” is 38 degrees, which the tsarist government rounded up to 40.” But no matter how many times we re-read this dissertation, we will not find a single word about the people’s favorite drink. In fact, the Russian government established a strength of 40 degrees for the convenience of calculating excise taxes levied on each degree back in 1843, when Mendeleev was barely 9 years old. And 38 degrees was the lower limit beyond which penalties for poor-quality products began.
Soon after his defense, Mendeleev already became an ordinary professor at the University. It was then, while working on a new textbook on inorganic chemistry, that he began to think about how the atomic weight of chemical elements and their other properties are related. For clarity, he created a separate card for each element, on which he wrote down brief information about it. The scientist carried a pack of these cards with him all the time and often sorted through them, playing them out like a cunning card game of solitaire. Which he had developed by February 1869.
True, it didn’t quite work out. Some elements did not quite correspond to the place in which the scientist placed them. In addition, the resulting table had three “holes”. Which Mendeleev “filled” with three fictitious elements - “eka-boron”, “eka-silicon” and “eka-aluminium”. All this allowed some of his colleagues to accuse the chemist of juggling and bending science to fit his “ridiculous theory.” The “Periodic Table” created by Mendeleev really took off only in 1875, when the French chemist Lecoq de Boisbaudran announced his discovery of a new element - gallium with a specific gravity of 4.7. Mendeleev noticed then that this element almost perfectly fits in place of “eka-aluminum”, with the only difference being that the latter had a calculated weight of around 5.9. The scientist reported this to his French colleague, who conducted more accurate experiments and found out that the real weight of gallium is 5.94. After this, the names of both chemists thundered throughout the world, and scientists rushed to feverishly clarify the old data, which increasingly corresponded to what the table gave, and look for the predicted elements. In 1879, “eka-boron” - “scandium” was discovered, and in 1885, “eka-silicon” - “germanium”. All these elements corresponded exactly to what was predicted for them by the new theory. Which by that time had already become generally accepted.
But, against the backdrop of such impressive scientific success, the scientist’s personal life suffered an increasingly obvious fiasco. Relations with his wife, which had previously been unimportant, by the end of the 1870s, Dmitry Ivanovich was completely upset. But on the old ashes the flame of a real love fire flared up. The culprit was the daughter of a Cossack from Uryupinsk, Anna Ivanovna Popova, who was often in the house. To her credit, it is worth saying that the lady did not at all seek to destroy the unit of society. As soon as Anna realized how far Dmitry’s feelings had gone, she tried to turn it all around, for which she simply left St. Petersburg for Italy. However, everything was too serious and, having learned about the escape of his beloved, the scientist quickly packed his things and rushed in pursuit. A month later he brought Anna Ivanovna back to St. Petersburg, and soon they started a new family. Over more than 20 years of marriage, Anna brought her husband four more children.
One should not think that Mendeleev was engaged only in chemistry. On the contrary, it is now difficult to find an area in which he has not proven himself to be a brilliant specialist. At the Imperial Academy of Sciences he was enrolled in the “physical” section. Among Russian oil workers, he was considered the most important specialist who proposed projects for the first oil pipelines and oil pumping stations. In 1879, he developed technological schemes for the first Russian plant for the production of machine oil.
In 1875, Mendeleev calculated the design of a stratospheric balloon with a sealed cabin for ascent to the upper layers of the atmosphere. And in the summer of 1887, he himself, as an aeronaut, rose above the clouds in the basket of a hydrogen-filled balloon in order to observe a solar eclipse. This was a real feat, because the scientist had no experience in aeronautics before. A professional pilot, Alexander Kovanko, was supposed to control the balloon, but it had rained the day before, the balloon got wet, became heavy and could not lift two people. After which the scientist dropped Kovanko out of the gondola, declaring that he would handle the ball himself. Under his control, the balloon rose to a height of almost 4 kilometers and flew more than 100 kilometers, after which Mendeleev made a completely successful landing. He himself wrote about this case: “... A significant role in my decision was played... by the consideration that we, professors and scientists in general, are usually thought of everywhere, that we speak, advise, but do not know how to master practical matters, that and we, like Shchedrin’s generals, always need a man to get things done, otherwise everything will fall out of our hands. I wanted to demonstrate that this opinion, perhaps fair in some other respects, is unfair in relation to natural scientists who spend their whole lives in the laboratory, on excursions and in general in the study of nature. We must certainly be able to master practice, and it seemed to me that it would be useful to demonstrate this so that everyone would one day know the truth instead of prejudice. Here was an excellent opportunity for this.” For this flight, the scientist was awarded a special medal from the Academy of Aerostatic Meteorology.
In the mid-1870s, Dmitri Mendeleev was on a commission to examine medium phenomena. Nowadays it would be called the “commission against pseudoscience.” Together with other famous scientists, he quite successfully exposed the machinations of a wide variety of mediums.
In the late 1870s, the scientist became interested in shipbuilding and drew up a project for an “experimental pool for testing ships.” And in the late 1890s, he was included in the commission for the construction of the world's first Arctic icebreaker. The icebreaking ship "Ermak" was launched in 1898.
Having become the Guardian Scientist of the General Chamber of Weights and Measures in 1892, he designed ultra-precise scales for weighing gaseous and solid substances. As a remarkable economist, at the end of the century he advised the Minister of Finance, Count Witte, on the issue of excise taxes and new customs law. In his works on demography, Mendeleev wrote: “The highest goal of politics is most clearly expressed in the development of conditions for human reproduction.” By the way, according to his calculations, by the middle of the 20th century, the population of Russia should have been 800 million people.
Finally, another widespread legend claims that Mendeleev was a master of the suitcase business and in his free time liked to create a couple of new suitcases. And although we don’t have a single suitcase left from him, this legend has some basis. The fact is that in his youth, at a time when work and money were tight, he really learned the basics of bookbinding and cartoning and often made folders and bindings himself for his own needs. Once, when I was already a serious scientist, I even made a small but durable cardboard bench, which has survived to this day. The scientist bought materials for this at Gostiny Dvor. It was then that he once heard a muffled dialogue behind him: “Who is this honorable gentleman?” - “Don’t you really know? This is the famous suitcase master Mendeleev.” The scientist had the imprudence to tell his friends about this anecdote, they told it to their acquaintances, and the story about the “great suitcase maker,” in a slightly modified form, began to circulate across the pages of newspapers and in the minds of ordinary people.
But the last legend - that the great chemist was not given the Nobel Prize due to a conflict with the Nobel family - may turn out to be true, although we do not have any documentary evidence of this. The scientist was nominated for the prize three years in a row - in 1905, 1906 and 1907. For the first time, it was surpassed by the German organic chemist Adolf Bayer.
In 1906, the Nobel Committee had already awarded Mendeleev the Prize, but the Royal Swedish Academy of Sciences reversed this decision. And here, quite possibly, the lobbying of the nephew of the childless Alfred Nobel and his main heir, Emanuel, who then headed the largest Russian oil corporation, the Nobel Brothers Partnership, had an impact. It is known that Mendeleev openly criticized the Nobels and accused them of a predatory attitude towards Russian oil. Therefore, purely theoretically, Emanuel, who had a certain weight in Nobel circles, could influence the fate of the prize. However, this seems unlikely: the Russian Swede Emanuel Nobel was not so vindictive. And we owe the very existence of the prize not least to him. Since the will in which it was mentioned was drawn up by the uncle with gross violations and could well have been contested by Emanuel in his favor. However, young Nobel recognized him, which almost put the family company, in which Alfred owned a third of the assets, on the brink of ruin.
Finally, a firm decision was made to award the Nobel Prize to the Russian chemist in 1907. However, according to the will, it could only be given to a living scientist. A Dmitry Ivanovich Mendeleev died on January 20, 1907.
Today, a city, towns, railway stations, metro stations, a volcano, a mountain peak, a glacier, a lunar crater, an asteroid are named after him; institutes, schools, scientific and non-scientific organizations, societies, congresses, magazines, plants and factories bear his name. And in 1955, American scientists included his name in the “Periodic Table” he created. Alfred Ghiorso, Burwell Harvey, Gregory Choppin and Stanley Thompson decided to name the 101 elements they discovered “Mendeleev” in honor of the legendary Russian scientist.
#Dmitriy Mendeleev
#story #greatRussian#Mendeleev #chemistry #educationDmitry Ivanovich Mendeleev was born in February 1834 in the city of Tobolsk, in the family of the director of a local gymnasium. His father, in the year of Dmitry’s birth, became blind in both eyes and, due to this, had to leave the service and go on a meager pension. Raising children and all concerns about a large family fell entirely on the shoulders of the mother, Maria Dmitrievna, an energetic and intelligent woman who, in order to improve the financial situation of the family, took over the management of her brother’s glass factory 25 km from Tobolsk. In 1848, the glass factory burned down, and the Mendeleevs moved to Moscow to live with their mother’s brother. In 1850, after much trouble, Dmitry Ivanovich entered the physics and mathematics department of the St. Petersburg Pedagogical Institute. In 1855, he graduated with a gold medal and was sent as a gymnasium teacher, first to Simferopol, and then to Odessa. However, Mendeleev did not remain in this position for long.
Already in 1856, he went to St. Petersburg and defended his master’s thesis on the topic “On specific volumes,” after which at the beginning of 1857 he was accepted as a private assistant professor in the department of chemistry at St. Petersburg University. 1859 - 1861 he spent on a scientific trip to Germany, at the University of Heidelberg, where he was fortunate to work under the guidance of outstanding scientists Bunsen and Kirchhoff. In 1860, Mendeleev took part in the first international chemical congress in Karlsruhe. Here he was keenly interested in the report of the Italian chemist Cannizzaro. “The decisive moment in the development of my thoughts about the periodic law,” he said many years later, “I consider 1860, the congress of chemists in Karlsruhe... and the ideas expressed at this congress by the Italian chemist Cannizzaro. I consider him to be my real predecessor, since the atomic weights he established provided the necessary fulcrum... The idea of a possible periodicity of the properties of elements with increasing atomic weight, in essence, already appeared to me internally..."
Upon returning to St. Petersburg, Mendeleev began vigorous scientific activity. In 1861, in a few months he wrote the first textbook on organic chemistry in Russia. The book turned out to be so successful that its first edition sold out in a few months and a second edition had to be made the following year. In the spring of 1862, the textbook was awarded the full Demidov Prize. With this money, Mendeleev made a trip abroad in the summer with his young wife Feozva Nikitichnaya Leshcheva. (This marriage was not very successful - in 1881 Mendeleev divorced his first wife, and in April 1882 he married the young artist Anna Ivanovna Popova.) In 1863 he received a professorship at the St. Petersburg Institute of Technology, and in 1866 - at St. Petersburg University, where he lectured on organic, inorganic and technical chemistry. In 1865, Mendeleev defended his doctoral dissertation on the topic “On the combination of alcohol with water.”
In 1866, Mendeleev acquired the Boblovo estate near Klin, with which his entire future life was then connected. Many of his works were written here. In his free time, he was very enthusiastic about farming on the experimental field he had established, where he tested various fertilizers. The old wooden house was dismantled over the course of several years, and a new stone one was built in its place. A model barnyard, dairy, and stable appeared. The threshing machine ordered by Mendeleev was brought to the estate.
In 1867, Mendeleev moved to St. Petersburg University as a professor of chemistry and was supposed to lecture on inorganic chemistry.
Having started preparing lectures, he discovered that neither in Russia nor abroad there was a course in general chemistry worthy of being recommended to students. And then he decided to write it himself. This fundamental work, called “Fundamentals of Chemistry,” was published in separate issues over several years. The first issue, containing an introduction, consideration of general issues of chemistry, description of the properties of hydrogen, oxygen and nitrogen, was completed relatively quickly - it appeared in the summer of 1868. But while working on the second issue, Mendeleev encountered great difficulties associated with the systematization and consistency of presentation of the material . At first he wanted to group all the elements he described by valence, but then he chose a different method and combined them into separate groups, based on the similarity of properties and atomic weight. Reflection on this question brought Mendeleev close to the main discovery of his life.
The fact that some chemical elements exhibit obvious similarities was not a secret to any chemist of those years. The similarities between lithium, sodium and potassium, between chlorine, bromine and iodine, or between calcium, strontium and barium were striking to anyone. In 1857, the Swedish chemist Lensen combined several “triads” by chemical similarity: ruthenium - rhodium - palladium; osmium - platinum ~ - iridium; manganese - iron - cobalt. Even attempts have been made to compile tables of the elements. The Mendeleev library contained a book by the German chemist Gmelin, who published such a table in 1843. In 1857, the English chemist Odling proposed his own version.
However, none of the proposed systems covered the entire set of known chemical elements. Although the existence of separate groups and separate families could be considered an established fact, the connection between these groups remained completely unclear.
Mendeleev managed to find it by arranging all the elements in order of increasing atomic mass. Establishing a periodic pattern required an enormous amount of thought from him. Having written on separate cards the names of elements indicating their atomic weight and fundamental properties, Mendeleev began to arrange them in various combinations, rearranging and changing places. The matter was greatly complicated by the fact that many elements had not yet been discovered at that time, and the atomic weights of those already known were determined with great inaccuracies. Nevertheless, the desired pattern was soon discovered. Mendeleev himself spoke in this way about his discovery of the periodic law: “Having suspected the existence of a relationship between elements back in my student years, I never tired of thinking about this problem from all sides, collecting materials, comparing and contrasting figures. Finally, the time came when the problem was ripe, when the solution seemed about to take shape in my head. As has always happened in my life, the premonition of an imminent resolution of the question that was tormenting me led me to an excited state. For several weeks I slept in fits and starts, trying to find that magical principle that would immediately put in order the entire pile of material accumulated over 15 years. And then one fine morning, having spent a sleepless night and despairing of finding a solution, I lay down on the sofa without undressing in the office and fell asleep. And in a dream, a table appeared to me quite clearly. I immediately woke up and sketched out the table I saw in a dream on the first piece of paper that came to hand.”
In February 1869, Mendeleev sent out to Russian and foreign chemists, printed on a separate sheet of paper, “An experiment on a system of elements based on their atomic weight and chemical similarity.” On March 6, at a meeting of the Russian Chemical Society, a message was read out about the classification of elements proposed by Mendeleev. This first version of the periodic table was quite different from the periodic table we were accustomed to from school.
The groups were arranged horizontally rather than vertically. The backbone of the table consisted of adjacent groups of alkali metals and halogens. Above the halogens was an oxygen group (sulfur, selenium, tellurium), above it was a nitrogen group (phosphorus, arsenic, antimony, bismuth). Even higher is the carbon group (silicon and tin, between which Mendeleev left an empty cell for an unknown element with an approximate mass of 70 a.u., which was later occupied by germanium with a mass of 72 a.u.) Above the carbon group were placed the boron and beryllium groups. Under the alkali metals there was a group of alkaline earth metals, etc. Several elements, as it turned out later, were placed out of place in this first version. Thus, mercury fell into the group of copper, uranium and gold - into the group of aluminum, thallium - into the group of alkali metals, manganese - into the same group with rhodium and platinum, and cobalt and nickel generally ended up in the same cell. But all these inaccuracies should not at all detract from the importance of the conclusion itself: by comparing the properties of the elements included in the vertical columns, one could clearly see that they change periodically as the atomic weight increases. This was the most important thing in Mendeleev’s discovery, which made it possible to connect together all the previously seemingly disparate groups of elements. Mendeleev quite correctly explained the unexpected disruptions in this periodic series by the fact that not all chemical elements are known to science. In his table, he left four blank cells, but predicted the atomic weight and chemical properties of these elements. He also corrected several inaccurately determined atomic masses of elements, and further research completely confirmed his correctness.
The first, still imperfect draft of the table was reconstructed in the following years. Already in 1869, Mendeleev placed the halogens and alkali metals not in the center of the table, but along its edges (as is done now). All other elements ended up inside the structure and served as a natural transition from one extreme to the other. Along with the main groups, Mendeleev began to distinguish subgroups (thus, the second row was formed by two subgroups: beryllium - magnesium - calcium - strontium - barium and zinc - cadmium - mercury). In the following years, Mendeleev corrected the atomic weights of 11 elements and changed the location of 20. As a result, in 1871, the article “Periodic Law for Chemical Elements” appeared, in which the periodic table took on a completely modern form. The article was translated into German and copies of it were sent to many famous European chemists. But, alas, Mendeleev did not expect from them not only a competent judgment, but even a simple answer. None of them appreciated the importance of the discovery he made. The attitude towards the periodic law changed only in 1875, when Lecoq de Boisbaudran discovered a new element - gallium, the properties of which strikingly coincided with the predictions of Mendeleev (he called this still unknown element equiluminum).
Mendeleev's new triumph was the discovery of scandium in 1879, and germanium in 1886, the properties of which also fully corresponded to Mendeleev's descriptions.
The ideas of the periodic law determined the structure of the “Fundamentals of Chemistry” (the last edition of the course with the periodic table attached to it was published in 1871) and gave this work amazing harmony and fundamentality. In terms of the power of influence on scientific thought, Mendeleev’s “Principles of Chemistry” can easily be compared with such outstanding works of scientific thought as Newton’s “Principles of Natural Philosophy,” Galileo’s “Conversations on the Two Systems of the World,” and Darwin’s “The Origin of Species.” All the vast factual material accumulated by this time on various branches of chemistry was presented here for the first time in the form of a coherent scientific system. Mendeleev himself spoke about the monograph textbook he created: “These “Fundamentals” are my favorite brainchild. They contain my image, my experience as a teacher and my sincere scientific thoughts.” The enormous interest that contemporaries and descendants showed in this book is entirely consistent with the opinion of the author himself. During Mendeleev’s lifetime alone, “Fundamentals of Chemistry” went through eight editions and was translated into major European languages.
In subsequent years, several more fundamental works on various branches of chemistry were published from the pen of Mendeleev. (His complete scientific and literary heritage is enormous and contains 431 published works.) In the mid-80s. he studied solutions for several years, the result of which was the “Study of Aqueous Solutions by Specific Gravity,” published in 1887, which Mendeleev considered one of his best works. In his theory of solutions, he proceeded from the fact that a solvent is not an indifferent medium in which it is rarefied a dissolving body, but an actively acting reagent that changes during the dissolution process, and that dissolution is not a mechanical process, but a chemical one. Proponents of the mechanical theory of the formation of solutions, on the contrary, believed that no chemical compounds arise during dissolution, and water molecules, combining in strictly defined proportions with the molecules of the substance, first form a concentrated solution, the mechanical mixture of which with water gives a diluted solution.
Mendeleev imagined this process differently - when combining with molecules of a substance, water molecules form many hydrates, some of which, however, are so fragile that they immediately disintegrate - dissociate. The products of this decomposition again combine with the substance, with the solvent and other hydrates, some of the newly formed compounds dissociate again, and the process continues until a mobile - dynamic - equilibrium is established in the solution.
Mendeleev himself was confident in the correctness of his concept, but, contrary to expectations, his work did not cause much resonance among chemists, since in the same 1887 two more theories of solutions appeared - Van't Hoff's osmotic and Arrhenius's electrolytic - which perfectly explained many of the observed phenomena. For several decades they completely established themselves in chemistry, pushing Mendeleev’s theory into the shadows. But in subsequent years it turned out that both the van't Hoff theory and the Arrhenius theory had a limited scope of application. Thus, Van't Hoff's equations gave excellent results only for organic substances. The Arrhenius theory (according to which decomposition - dissociation - of electrolyte molecules (salts, acids and alkalis) into positively and negatively charged ions occurs in a liquid) turned out to be valid only for weak solutions of electrolytes, but did not explain the main thing - how and due to what forces the splitting occurs the strongest molecules when they enter water. After Mendeleev’s death, Arrhenius himself wrote that the hydrate theory deserves detailed study, because it is it that can provide the key to understanding this, the most difficult issue of electrolytic dissociation. Thus, Mendeleev’s hydration theory, along with the solvate theory of van’t Hoff and the electrolytic theory of Arrhenius, has become an important part of the modern theory of solutions.
Mendeleev's works received wide international recognition. He was elected a member of the American, Irish, Yugoslav, Roman, Belgian, Danish, Czech, Krakow and many other academies of sciences, and an honorary member of many foreign scientific societies. Only the Russian Academy of Sciences voted him out in the elections of 1880 due to some kind of internal intrigue.
After retiring in 1890, Mendeleev took an active part in the publication of the Brockhaus and Efron Encyclopedic Dictionary, then for several years he was a consultant in the gunpowder laboratory at the Naval Ministry. Before this, he had never been specifically involved in explosives, but after conducting the necessary research, in just three years he developed a very effective composition of smokeless gunpowder, which was put into production. In 1893, Mendeleev was appointed custodian (manager) of the Main Chamber of Weights and Measures. He died in February 1907 from pneumonia.